Abstract
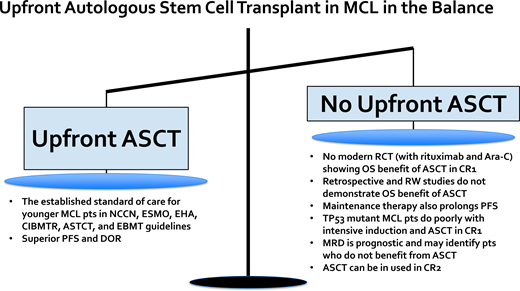
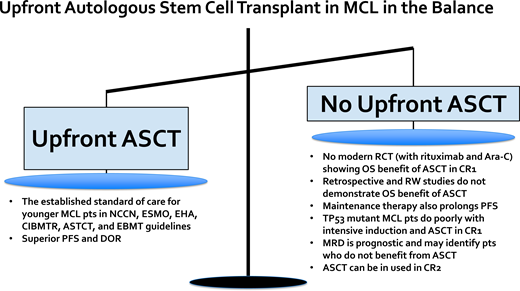
Up-front autologous stem cell transplantation (ASCT) is the established standard of care for younger, transplant-eligible MCL patients and is associated with a prolonged progression-free survival (PFS) benefit. However, there is no randomized controlled trial data, with therapy including rituximab and cytarabine, that has established a PFS and overall survival (OS) benefit with ASCT in the modern era. Multiple retrospective studies have failed to identify an OS benefit associated with ASCT in younger MCL patients. The high-risk patient subgroup with evidence of baseline TP53 mutation has a dismal outcome with intensive chemoimmunotherapy followed by ASCT, thus up-front ASCT is not optimal for this patient subset. Ongoing randomized clinical trials will help to clarify the role of up-front ASCT in the future. For example, the ongoing European MCL Network Triangle study incorporating ibrutinib into chemoimmunotherapy induction and maintenance with and without ASCT will help define the role of ASCT in the era of novel biologically targeted agents (ClinicalTrials.gov identifier: NCT02858258). Additionally, minimal residual disease (MRD) assessment is a powerful prognostic tool in MCL, and the ongoing Eastern Cooperative Oncology Group-American College of Radiology Imaging Network E4151 study is comparing maintenance rituximab alone vs ASCT consolidation in MCL patients who achieve remission and MRD-undetectable status post induction (ClinicalTrials.gov identifier: NCT03267433). ASCT remains a highly efficacious initial therapy for younger MCL patients; however, ultimately the decision to pursue ASCT requires discussion of risks vs benefits, incorporating patient preferences and values.
Learning Objectives
Understand the data that support and challenge the use of ASCT in first remission in younger, fit MCL patients
Understand that the presence of TP53 mutation is associated with a poor response to standard chemoimmunotherapy, including ASCT
Review the use of novel therapies and MRD assessment in clinical trials that will help define the future role of ASCT in the modern era
CLINICAL CASE 1
A 55-year-old otherwise healthy woman presented with intermittent left lower quadrant abdominal pain. A computed tomographic scan of the abdomen and pelvis revealed intussusception of the descending colon, and a colonoscopy showed a 5-cm partially obstructing ulcerated mass in the descending colon. Biopsy of the colonic mass was consistent with mantle cell lymphoma (MCL). Neoplastic cells expressed by immunohistochemistry (IHC) included CD20, CD5, BCL2, and cyclin D1 but did not express TP53. Ki-67 was 25% to 40%. Mutational analysis revealed no TP53 mutation. A positron emission tomographic (PET) scan revealed diffuse fluorodeoxyglucose (FDG)-avid lymphadenopathy and FDG avidity throughout the gastrointestinal (GI) tract. The CBC and lactate dehydrogenase were normal, consistent with the low-risk clinical Mantle Cell Lymphoma International Prognostic Index (MIPI) score. The patient underwent 4 cycles of RDHAX chemotherapy (rituximab, dexamethasone, cytarabine, and oxaliplatin) and achieved a complete remission (CR). She then received ASCT consolidation followed by maintenance rituximab.
The standard of care frontline therapy for MCL patients is chemoimmunotherapy. For younger MCL patients (≤65 years) who are transplant eligible, a widely accepted treatment approach consists of 3 phases: (1) cytarabine-containing induction chemoimmunotherapy, (2) ASCT, and (3) maintenance rituximab. This treatment paradigm is supported by various national and international guidelines and expert panels, including the National Comprehensive Cancer Network, American Society of Transplantation and Cellular Therapy, Center for International Blood and Marrow Transplant Research, European Society for Blood and Marrow Transplantation, European Society for Medical Oncology, and European Hematology Association.1-4
The pivotal study that established the role of consolidative ASCT in first remission for fit MCL patients was the open-label, multicenter, randomized phase 3 trial performed by the European Mantle Cell Lymphoma Network enrolling patients between September 1996 and March 2004.5 In this study, patients with advanced-stage MCL aged 65 years or younger were randomized to receive either ASCT or interferon alfa (IFNα) maintenance therapy after cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP)-like induction therapy with or without rituximab (ie, R-CHOP). In the initial report with a median follow-up of 25 months, patients in the ASCT arm vs the IFNα arm had a significantly longer progression-free survival (PFS; median of 39 vs 17 months; P = .0108) but no significant difference in overall survival (OS). Recently published long-term results with a 14-year median follow-up continued to demonstrate a PFS benefit and also showed an OS benefit favoring ASCT consolidation vs IFNα maintenance.6 Building upon this approach, the treatment paradigm for younger, fit MCL patients has been further refined to include rituximab and cytarabine in the initial induction regimen and to include rituximab maintenance post ASCT.7,8 The therapeutic importance of high-dose cytarabine was established by an open-label, phase 3 trial from the European Mantle Cell Lymphoma Network in which MCL patients aged 65 years or younger were randomized to receive R-CHOP vs alternating R-CHOP and R-DHAP (rituximab plus dexamethasone, high-dose cytarabine, and cisplatin) for 6 cycles followed by ASCT.7 After a median follow-up of 6 years, the time to treatment failure (TTF) was significantly longer in the R-CHOP/R-DHAP group vs the R-CHOP-alone group (5-year TTF was 65% vs 40%, respectively), thus supporting the inclusion of cytarabine as part of initial induction chemoimmunotherapy. In the initial report, there was no OS difference between the R-DHAP-containing vs R-CHOP-alone treatment arms, but with 5 additional years of follow-up, when adjusted for MIPI score without and with Ki-67, OS was significantly superior in the R-DHAP arm (hazard ratio [HR], 0.74; P = .038 and 0.60; P = .0066).9 The LYMA study established rituximab maintenance post ASCT as a standard of care.8 In this study, MCL patients aged 65 years or younger received 4 courses of R-DHAP (any platinum) followed by ASCT consolidation and then were randomized to receive rituximab maintenance vs observation post transplant. The PFS and OS at 4 years were superior in the rituximab maintenance vs observation group (PFS, 83% vs 64%; P < .001; OS, 89% vs 80%; P = .04), respectively.
Multiple phase 2 studies have demonstrated long-term durable remissions with cytarabine-containing induction chemoimmunotherapy followed by ASCT consolidation (Table 1).7,8,10-16 For example, with a median follow-up of 11.4 years, the second Nordic MCL trial (MCL2) with patients under 66 years of age who received alternating courses of R-maxi-CHOP (dose-intensified rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) and high-dose cytarabine (HIDAC) and ASCT consolidation was associated with a median OS and PFS of 12.7 and 8.5 years, respectively.10,11 Although there was a continuous pattern of relapse over time, for low-risk MIPI patients the median PFS was over 12 years with the Nordic MCL2 treatment approach. This prolonged initial duration of response likely has a greater impact on OS in younger vs older MCL patients given the fewer treatment-related toxicities and competing health risks. Given the successive shortening of remission length with subsequent lines of therapy in MCL, optimizing initial treatment is of value.17 Another reason to utilize ASCT in frontline is that MCL can evolve over time, and the acquisition of high-risk mutations, genomic instability, and/or transformation to blastoid or pleomorphic disease are increasingly common in the relapsed setting and often associated with disease refractory to standard chemoimmunotherapy.18 An extended “treatment-free” interval after frontline therapy also affords time for advances in the field to develop; for example, between 2013 and 2022, 6 novel therapies were US Food and Drug Administration or National Comprehensive Cancer Network approved for R/R MCL, including lenalidomide, ibrutinib, acalabrutinib, zanubrutnib, venetoclax, and brexucabtagene autoleucel.19-24 In summary, frontline intensive induction followed by ASCT and rituximab maintenance for younger, fit MCL patients is a highly efficacious treatment approach and associated with a prolonged PFS benefit.
CLINICAL CASE 2
A 64-year-old man with a history of well-controlled hypertension and hyperlipidemia presented with fatigue and drenching night sweats. A PET scan revealed diffuse lymphadenopathy and splenic involvement. Biopsy of a lymph node revealed MCL. Neoplastic cells expressed CD20, CD5, BCL2, and cyclin D1 and did not express TP53 by IHC. Ki-67 was 20%. Mutational analysis revealed ATM mutation alone. Bone marrow (BM) biopsy and aspiration showed 50% to 70% involvement with MCL. Labs revealed mild anemia and thrombocytopenia. The MIPI score was intermediate risk. The patient expressed a strong desire to receive outpatient chemoimmunotherapy, balked at the idea of temporarily relocating to New York City for an ASCT, and wanted to maintain his quality of life to continue working and caring for his elderly parent. The patient received treatment with bendamustine and rituximab for 6 cycles followed by rituximab maintenance in his local community.
Although ASCT in CR1 established the standard of care for younger MCL patients, it is not the only acceptable treatment. It is becoming increasingly clear based on retrospective and real-world data that only a fraction of younger patients with MCL receive up-front ASCT despite national and international guidelines supporting its use. Recently published data from the Flatiron Health database describing treatment patterns for MCL patients treated in predominantly community-based practices from 2011 to 2021 showed that only approximately 1 in 4 patients under 65 years of age received an up-front ASCT.25 Data from the Center for International Blood and Marrow Transplant Research database and estimated MCL incidence rates from the National Cancer Institute's Surveillance, Epidemiology, and End Results database between 2000 and 2017 showed that in patients aged 65 years or younger, ASCT utilization increased from 5.4% in 2001 to a peak of 22.3% in 2013 and plateaued at 18.1% in 2017.26 In addition, an analysis from the National Cancer Database estimated ASCT utilization to be approximately 30% for patients aged 60 years or younger.27 Rates of ASCT utilization for younger patients appear higher at US academic medical centers (67%) and in certain geographies, like Sweden and Denmark.28-30
A number of factors may contribute to why ASCT utilization rates are lower than anticipated in younger MCL patients. In the Surveillance, Epidemiology, and End Results database study, private health insurance, care at an academic/research medical center, and geographic location were significantly associated with ASCT utilization.27 A Swedish registry study found that MCL patients who had never married, were divorced, or had a lower educational level underwent transplantation less often.29 In the multicenter retrospective study that characterized outcomes and treatment patterns for younger MCL patients treated at US academic medical centers, the reasons for not selecting ASCT consolidation included physician choice, 67%; patient preference, 18%; other reason (eg, mobilization failure), 3%; and unknown, 12%.28 Overall, factors that influence whether up-front ASCT is pursued include patient and/or physician preference, academic vs community-based care, and socioeconomic factors.
One of the reasons why physicians may not recommend up-front ASCT in MCL is because of emerging data that fail to convincingly demonstrate an associated OS benefit. The survival advantage previously described with up-front ASCT may be diminished in the context of improved frontline induction programs (including rituximab and cytarabine) with incorporation of maintenance post induction, as well as with improved salvage therapies for relapsed MCL, such as Bruton's tyrosine kinase inhibitors, CD19-directed chimeric-antigen receptor T-cell therapy, and other novel agents.
One critical question that underlies the controversy surrounding ASCT in MCL is: Would the same PFS and OS benefit of ASCT reported in the only randomized pivotal study from the European Mantle Cell Lymphoma Network remain in the current era given access to rituximab, cytarabine, and better salvage therapies? To explore this question, a post hoc subgroup exploratory analysis was performed from the pivotal study, focusing on patients who received rituximab-containing induction regimens. Neither PFS nor OS were significantly different between the ASCT vs IFNα maintenance groups, with a median PFS of 3.4 years vs 1.7 years (P = .087) and a median OS of 9.6 years vs 5.5 years (P = 0.68), respectively.6 However, this post hoc subgroup analysis included a small number of patients who received rituximab-containing induction therapy (41 patients who received ASCT consolidation and 27 patients who received IFNα maintenance) and thus was underpowered to detect a significant difference between the 2 approaches.6 Another important retrospective study aiming to assess the benefit of ASCT in the rituximab era was a multicenter analysis from several North American academic medical centers that included 1029 patients aged 65 years or younger.28 In this study, 64% of patients (n = 657) underwent ASCT consolidation, and after a median follow-up of 76 months, ASCT was associated, using a multivariable regression analysis, with a statistically significant improvement in PFS and a trend toward improved OS. In a propensity score-weighted analysis aimed to address confounders, the median PFS was more prolonged with ASCT (78 vs 48.5 months), but an improvement in OS was not observed. In addition, several real-world analyses also do not show a clear survival benefit to be associated with ASCT. For example, the Flatiron Health database examined ASCT-eligible patients (<65 years who were alive and did not initiate subsequent treatment within 6 months of first-line treatment) and found no significant improvement in real-world time-to-next-treatment or OS with ASCT (vs without).25 In a real-world study of patients diagnosed in Sweden between 2007 and 2017, for patients under 70 years of age there was no significant difference in OS with intensified MCL2 protocol or R-CHOP compared to bendamustine- rituximab.31
Additionally, it may be possible to consider the deferral of ASCT to the relapsed/refractory (R/R) setting. There are data to suggest that ASCT can be effective in CR2, although it is unclear whether outcomes are equivalent to up-front ASCT.32,33
In light of these data, for the patient in Clinical Case 2 I recommend discussing the outcome data that support up-front ASCT, the emerging data that challenge this therapeutic strategy in the modern era, and the short- and long-term side effects of an intensive approach vs other options. Ultimately, the oncologist and the patient can collaborate to devise a care plan based on clinical evidence that balances risks and expected outcomes with patient preferences and values (Table 2). For this patient, the significant time commitment and need for hospitalization, the cost of travel to an urban transplant center, and the potential toxicities associated with ASCT represented an undue burden not offset by the likely prolonged remission duration post transplant, particularly given the potential lack of OS benefit associated with up-front ASCT in the modern era.
CLINICAL CASE 3
A 40-year-old otherwise healthy woman presented with worsening left lower quadrant pain requiring opioid therapy, and a computed tomographic scan of the abdomen and pelvis revealed a bulky retroperitoneal mass measuring 12 cm, causing left-sided hydronephrosis. Biopsy of the mass revealed MCL, blastoid variant. Neoplastic cells expressed CD20, CD5, BCL2, and cyclin D1 by IHC. TP53 expression by IHC was not evaluated. Ki-67 was 60% to 70%. Mutational analysis was sent and pending at the time of the initial presentation. A PET scan revealed diffuse FDG-avid lymphadenopathy, including a bulky lymph node conglomerate in the retroperitoneum, and BM biopsy and aspiration showed 10% to 15% involvement with MCL. The MIPI score was high risk. The patient was admitted for pain control and the initiation of RDHAX with tumor lysis monitoring. She achieved a CR after 4 cycles. During therapy, mutational profiling results returned and revealed the presence of a missense mutation in the TP53 gene (R249T). Although she had been scheduled for ASCT consolidation, this was aborted, and the patient proceeded to receive rituximab maintenance.
The presence of TP53 mutation is associated with a very poor prognosis when treated with conventional chemoimmunotherapy, including ASCT consolidation. In a pooled analysis from the Nordic MCL2 and MCL3 clinical trials, the prognostic value of TP53 mutation at baseline was assessed in 183 patients (of the total 320 enrolled) who had available DNA from diagnostic BM samples. In the MCL2 and MCL3 studies, patients under 66 years of age with stage II to IV disease who were transplant eligible received alternating R-maxi-CHOP and R-high-dose-cytarabine followed by ASCT.34 TP53 mutations were observed in 11%, del17p in 16%, NOTCH1 mutations in 4%, and CDKN2A mutations in 20%. All these genetic subgroups, in addition to the MIPI, MIPI-c, blastoid morphology, and Ki-67% over 30% groups, were each associated with inferior survival on univariable analysis. However, on multivariable analysis, only the MIPI-c high-risk group (HR, 2.6; P = .003) and TP53 mutation (HR, 6.2; P < .0001) retained independent prognostic significance for OS. Patients with TP53 mutation had a percentage of Ki-67 greater than 30%, blastoid morphology, and a high-risk MIPI. The outcomes for patients with TP53 mutation were dismal, with a median PFS of 0.9 years and a median OS of 1.8 years. TP53 mutation with or without del17p was associated with inferior outcomes compared to the presence of del17p alone. Increased TP53 expression by IHC also has prognostic value in MCL and is highly correlated with the presence of TP53 mutation.35-37 Given the minimal benefit and potential toxicity of ASCT in this high-risk subset of patients, ASCT consolidation is not recommended in MCL patients with evidence of TP53 mutation.
There exists no optimal frontline treatment approach for this high-risk subset of patients, and clinical trial enrollment is highly recommended. Emerging treatment options for high-risk patients include combinations of biologically targeted therapies, such as dual inhibition of Bruton's tyrosine kinase and BCL2 in conjunction with anti-CD20 monoclonal antibody therapy, which has been, for example, investigated in the OASIS and BOVen studies.38,39 Encouragingly, brexucabtagene autoleucel (KTE-X19), an anti-CD19 autologous CAR-T cell US Food and Drug Administration approved for R/R MCL, has shown promising efficacy even among high-risk MCL patients with elevated proliferative indices, blastic or pleomorphic morphology, and TP53 mutation.24,40 In the future, novel treatment options, including anti-CD19 chimeric-antigen receptor T-cell therapy or CD20/CD3 bispecific antibody therapy, will likely be incorporated into clinical trials in earlier lines, particularly for high-risk patients.
CLINICAL CASE 1 (Continued)
We return again to the 55-year-old woman with MCL, Ki-67 25% to 40%, TP53 wild-type stage IV MIPI low-risk disease involving the GI tract who received 4 cycles of RDHAX and achieved a CR. In this case, the patient enrolled in the ECOG-ACRIN EA4151 trial titled Rituximab With or Without Stem Cell Transplant in Treating Patients With Minimal Residual Disease-Negative Mantle Cell Lymphoma in First Complete Remission (ClinicalTrials.gov identifier: NCT03267433). At the end of induction chemotherapy, she was minimal residual disease (MRD) undetectable by an immunosequencing assay (clonoSEQ®) and was randomized to receive rituximab maintenance alone (ASCT omitted).
The lack of clear OS benefit associated with ASCT in MCL in the modern era has paved the way for the development of clinical trials testing alternate therapeutic strategies omitting ASCT. These clinical trials have tested different approaches including the use of maintenance therapy post induction, the incorporation of novel agents, and the use of MRD assessment to develop risk-adapted treatment paradigms. For example, the E1405 study utilized a modified rituximab, hyperfractionated cyclophosphamide, doxorubicin, vincristine, and dexamethasone (R-HCVAD) regimen incorporating bortezomib with maintenance rituximab, and the primary end point of the study was overall response rate (ORR) to this combination.41 The ORR was 95%, and a CR was achieved in 68% of patients. Interestingly, in this study patients could receive either maintenance rituximab or ASCT per physician and patient preference, and with a median follow-up of 4.5 years, no substantial difference in PFS or OS was found between patients treated with maintenance rituximab (n = 44) vs ASCT (n = 22). Although this was only hypothesis generating given the lack of a randomized study design, short follow-up time, and small sample sizes, this study set the stage for future clinical trials to more definitively compare maintenance rituximab and ASCT post induction in a larger randomized context.
The prognostic role of MRD assessment, utilizing different assays including flow cytometry, reverse-transcription polymerase chain reaction (PCR)–based techniques, and immunosequencing platforms, has been well established in MCL.42 MRD status post induction chemotherapy and pre-ASCT has been shown to be an important predictor of PFS and a potential biomarker for risk-adapted treatments.43,44 Thus, the ongoing EA4151 study is comparing maintenance rituximab alone vs ASCT consolidation in MCL patients who achieve a remission and an MRD-undetectable status post induction. The primary end point of this study is 6-year OS. The study has the potential to establish an MRD-guided treatment strategy, allowing for therapy de-escalation in selected MRD-undetectable patients.
Other studies have incorporated novel agents, such as ibrutinib, into up-front regimens. The phase 2 WINDOW-1 study minimized chemotherapy for previously untreated MCL patients 65 years or younger.45 Patients were initially treated with ibrutinib and rituximab, and after achieving CR, patients received R-HCVAD alternating with methotrexate/cytarabine for 4 cycles. The ORR was 98% (n = 131 patients treated), the 3-year estimated PFS was 79%, and the 3-year OS was 95%. Additionally, in the ongoing European MCL Network Triangle study, transplant-eligible patients under 65 were randomized to 1 of 3 arms (rituximab maintenance can be added to all arms): (1) R-CHOP/R-DHAP induction with ASCT, (2) R-CHOP plus ibrutinib/R-DHAP with ASCT and then 2 years of ibrutinib maintenance, and (3) R-CHOP plus ibrutinib/R-DHAP followed by ibrutinib maintenance for 2 years without ASCT (ClinicalTrials.gov identifier: NCT02858258).46 The primary end point is superiority of 1 of the 3 study arms by investigator-assessed failure-free survival. This study will determine the value of ibrutinib during induction and maintenance and determine if ASCT can be omitted with the addition of ibrutinib.
Conclusion
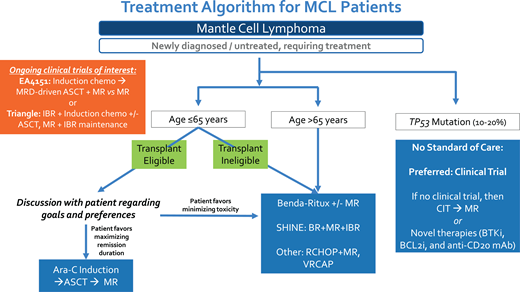
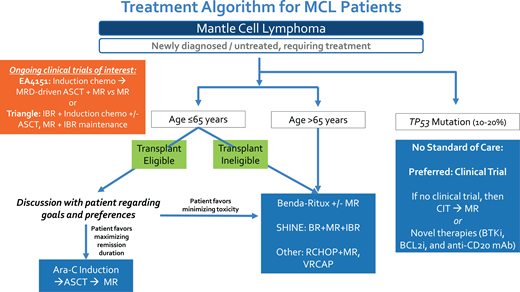
All 4 clinical cases presented highlight the evolving role of up-front ASCT in MCL, and I have summarized my approach in Figure 1. While up-front ASCT remains a well-established and highly effective initial therapeutic strategy for younger MCL patients, there are subgroups of patients in whom this approach is not warranted, such as patients with TP53 mutation. Although ASCT consolidation has been utilized in patients older than 65 years who are fit without significant comorbidity, I do not support this approach since the grand majority of clinical trial data supporting up-front ASCT in MCL have focused on younger patients, and the already uncertain OS benefit of ASCT is even less well established in older patients who have an increased risk for transplant-associated toxicities and competing risks for survival with advanced age.47,48 Ultimately, after discussion of benefits vs risks, it is critical to develop individualized treatment plans that incorporate patient preferences and values. Key clinical trials, including EA4151 and the European Triangle study, will help to further clarify the role of ASCT in the future.
Conflict-of-interest disclosure
Anita Kumar: research funding: Abbvie Pharmaceuticals, Adaptive Biotechnologies, Celgene, Pharmacyclics, Seattle Genetics, Astra Zeneca, Loxo Oncology/Lily; honoraria: Celgene, Genentech, Kite Pharmaceuticals, Loxo Oncology/Lily, Astra Zeneca; advisory board: Celgene, Genentech, Kite Pharmaceuticals, Loxo Oncology/Lily, Astra Zeneca.
Off-label drug use
Anita Kumar: nothing to disclose.