Abstract
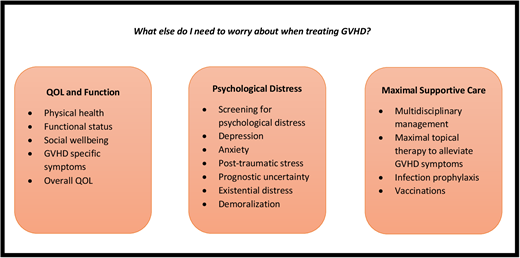
Graft-versus-host disease (GVHD) is the main cause of morbidity and mortality in allogeneic hematopoietic stem cell transplant survivors. Patients with acute and chronic GVHD often endure substantial symptom burden and quality of life (QOL) and functional impairments. Living with GVHD affects multiple domains of patient-reported QOL, physical functioning, and psychological well-being. Patients describe living with GVHD as a life-altering “full-time job” requiring unique knowledge, personal growth, and resilient coping strategies. Managing the supportive care needs of patients living with GVHD must include (1) monitoring of patient-reported QOL and symptom burden; (2) routine screening for psychological distress and implementing therapeutic strategies to treat depression, anxiety, and posttraumatic stress symptoms; (3) a systematic review of care needs by a multidisciplinary team experienced in managing transplant-related complications and organ-specific GVHD symptoms; and (4) ensuring optimal prevention and management of infection complications in this highly immunocompromised population. Improving the QOL in patients with GVHD requires a multidisciplinary approach with emphasis on aggressive symptom management, psychological coping, and promoting physical activity and rehabilitation in this population living with immense prognostic uncertainty and struggling to adapt to this difficult and unpredictable illness.
Learning Objectives
Understand the impact of GVHD on quality of life, functional status, symptom burden, and psychological distress in allogeneic HCT survivors
Develop strategies to address the supportive care needs of patients with GVHD
CLINICAL CASE
Emma is a 39-year-old woman, a middle school teacher, who was initially diagnosed with acute myeloid leukemia and achieved a complete remission after induction chemotherapy. Subsequently, she underwent a myeloablative matched unrelated donor peripheral stem cell transplantation during a 4-week hospitalization. Two weeks after discharge, she developed diarrhea, prompting a hospital readmission. She was diagnosed with acute skin and gastrointestinal graft-versus-host disease (GVHD) and initiated first-line therapy with methylprednisolone 2 mg/kg/d. She had a complete response within 16 days of therapy and was discharged from the hospital 3 weeks later. Due to prolonged corticosteroid exposure, Emma developed steroid myopathy and weakness, and she also struggled with some lower extremity edema during the first few months following her rehospitalization.
The supportive care needs of patients with acute GVHD
Acute GVHD is a common complication of allogeneic hematopoietic stem cell transplant (HCT), which has a significant impact on patient-reported quality of life (QOL), physical functioning, and clinical outcomes.1,2 Classic acute GVHD typically occurs within the first 100 days posttransplant and predominantly affects the skin, liver, and gastrointestinal tract.1,2 Recent advances in the assessment and management of acute GVHD rely on identification and risk stratification of patients based on clinical staging of the disease and blood biomarkers.3-5 Despite these advances, there are substantial gaps in our understanding of the supportive care needs of this population, particularly when it comes to their QOL and overall functioning.6 Patients with acute GVHD struggle with substantial symptom burden, including nausea, anorexia, diarrhea, and decline in functional status.6 As in Emma's case, treatment of acute GVHD typically involves high-dose corticosteroid therapy along with other immunosuppressive agents, which may further increase the risk of complications and symptoms such as infections and weakness.
Studies comprehensively examining the supportive care needs of patients with acute GVHD are lacking in part due to (1) the lack of a valid and reliable tool specific for symptoms of acute GVHD; (2) the need for frequent patient-reported assessments, which can place substantial burden on this acutely ill population; and (3) lack of robust studies correlating the objective response criteria with clinically meaningful changes in QOL and acute GVHD symptoms.6 Nonetheless, multiple studies have shown that acute GVHD is associated with a decline in patient-reported QOL, physical functioning, role functioning, social functioning, mental health, and general health.7-11 As highlighted in Emma's clinical case, patients with acute GVHD often spend prolonged periods in the hospital, which further contributes to their social isolation and psychological distress. Given the extent of trauma and isolation these patients experience during their illness course, studies are also needed to examine their existential distress and demoralization.
When caring for patients with acute GVHD, clinicians should at minimum screen for psychological distress. Prior work has shown the feasibility of patient-reported outcomes monitoring and psychosocial distress screening in allogeneic HCT recipients.12,13 Studies integrating serial patient-reported outcomes monitoring during the acute GVHD course will help clinicians guide clinical care in addressing the unmet needs of this population, as well as monitor potential response to therapy. In addition, clinical trials focused on the management of acute GVHD must also incorporate serial patient-reported outcome assessments that allow for robust examination of response to therapy and complications related to prolonged steroid use such as weakness, functional decline, fatigue, insomnia, and psychological distress. Moreover, maximal supportive care measures, including topical treatments for skin GVHD as well as antidiarrheal therapy for patients with gastrointestinal GVHD, should be implemented routinely in clinical practice. Given the potential risk of functional decline, physical therapy and rehabilitation are also essential components to maximize the QOL and functioning of patients with acute GVHD.14,15 Cumulative exposure to high-dose corticosteroids is associated with adverse side effects, including infection complications, avascular necrosis, osteoporosis, edema, and steroid myopathy.16 In fact, the incidence of steroid myopathy in patients with acute GVHD is as high as 41%.17 Studies have shown that patients with acute GVHD have baseline impairments in their function, which are worsened within 14 days of receiving corticosteroid therapy.14 These findings underscore the potential need for early supervised consultation with physical therapy, occupational therapy, and physical medicine and rehabilitation to closely monitor and intervene to improve physical functioning in this population.14 As in Emma's case, patients can also develop some lower extremity edema and fluid retention due to corticosteroid use, poor nutrition and hypoalbuminemia, and their inflammatory state from acute GVHD. Prompt management of these complications and close attention to nutritional needs in this population can improve patients' overall QOL and functioning. Notably, recent studies have shown an association between the gut microbiota and the risk of developing acute GVHD as well as overall transplant outcomes.18 Future therapies for acute GVHD, including probiotics, nutritional supplements, and fecal microbiota transplantation, are currently being tested in clinical trials.18
In a randomized clinical trial, the integration of specialty palliative care has been shown to improve a wide range of patient-reported outcomes, including QOL, symptom burden, fatigue, depression, anxiety, and posttraumatic stress symptoms both acutely during HCT hospitalization and up to 6 months post-HCT.19,20 Although these data are not specific to patients with acute GVHD, admission for acute GVHD is a reasonable trigger for specialty palliative care given the role of palliative care in managing complex symptoms, facilitating effective coping, cultivating prognostic awareness, and addressing the psychological and existential distress in patients living with a serious illness. Although comprehensive geriatric assessments have not been tested in patients with acute GVHD, they focus on numerous domains that are affected by patients with acute GVHD. A comprehensive geriatric assessment model of care that incorporates a focus on functional capacity, cognition, mood, polypharmacy, social support, financial concerns, and nutrition, as well as overall goals of care, may be a beneficial framework to address the needs of this population.
CLINICAL CASE (continued)
Emma recovered from her acute GVHD symptoms and continued to make progress toward her HCT recovery. At 8 months post-HCT, Emma developed symptoms of chronic GVHD affecting her skin, mouth, eyes, and vagina while tapering her immunosuppression therapy. Overall, she developed moderate chronic GVHD based on the number of organ systems involved. She received treatment with systemic corticosteroids, tacrolimus, ruxolitinib, and ibrutinib. Given the severity of her symptoms, Emma has not been able to return to work. She also struggles to manage her daily routine with topical therapies for her oral, ocular, skin, and vaginal GVHD.
QOL and symptom burden in chronic GVHD
Chronic GVHD is a debilitating immunologic syndrome that attacks multiple organs and is the major cause of post-HCT morbidity and mortality.21-25 Patients with chronic GVHD struggle to manage their illness, which often results in substantial physical symptoms, functional limitations, and impaired QOL.9,21,22,26,27 Treatment of chronic GVHD, such as corticosteroids, is limited in efficacy and has significant toxicities, thus contributing to the unpredictable trajectory of this illness and its unrelenting course.27 Studies have shown that the severity of chronic GVHD is associated with the extent of QOL, physical, and functional impairments seen in this population.28 The Lee Symptom Scale is a reliable and valid measure for chronic GVHD symptoms that correlates with National Institutes of Health criteria as well as patient-reported chronic GVHD severity.29
Given the global and multidimensional impact of chronic GVHD in allogeneic HCT survivors, patients with chronic GVHD should be reviewed by a team experienced in managing transplant-related complications and the supportive care needs of this population.30 Furthermore, transplant centers should establish a clinical network of specialists with an interest in chronic GVHD to allow for multidisciplinary management.30 Assessment of QOL and chronic GVHD symptoms is recommended for all patients living with chronic GVHD.28 Improving QOL in this population requires a multidisciplinary approach with a focus on aggressive symptom management, psychological coping, and promoting physical activity and rehabilitation.30
Psychological distress in patients with chronic GVHD
Given the physical symptom burden and uncertainty about the illness course in patients with chronic GVHD, psychological distress is prevalent in this population, with 50% and 30% of patients with chronic GVHD reporting depression and anxiety symptoms, respectively.31,32 Notably, self-reported depression symptoms have been associated with lower survival in patients with chronic GVHD.28 In prior studies, the use of adaptive coping strategies was associated with lower psychological distress in patients with chronic GVHD.29 Coping style is a modifiable construct that, if altered to be more adaptive in the context of a chronic medical stressor, could potentially change the trajectory of QOL and psychological distress in patients with chronic GVHD.32 Therefore, psychological interventions, such as cognitive behavioral therapy, to promote adaptation and enhance effective coping have a promising potential for improving QOL and mood in this population.33-39
Similar to acute GVHD, screening for psychosocial distress and prompt referral to supportive care services such as psychology, psychiatry, and/or social work for proactive management of depression and anxiety are critical to optimizing the quality of care for patients with chronic GVHD.31,32 In addition to therapy with a licensed clinical social worker or psychologist, patients may benefit from a pharmacologic approach for mood management with antidepressants or anxiolytics. Financial distress can also be a cause of psychological distress in patients living with chronic GVHD and dealing with an unrelenting illness. Although studies have focused on financial distress in allogeneic HCT recipients,40 future assessment of the extent of financial distress and toxicity is needed for patients with chronic GVHD along with consideration for financial navigation programs and other evidence-based interventions to reduce financial toxicity and psychological distress in this population.
Organ-specific supportive care management for chronic GVHD
Management of organ-specific issues is a core component of optimal supportive GVHD care that entails topical therapy and other interventions directed at organ specific–control of symptoms.30,41 The National Institutes of Health Consensus Development Project and the British Society for Bone Marrow Transplantation have both instituted recommendations for the supportive care and management of organ-specific complications of chronic GVHD.42 We review the specific therapies for the most common chronic GVHD symptoms, including skin, oral, ocular, and genital manifestations of chronic GVHD.
Skin chronic GVHD
Supportive care for skin chronic GVHD focuses on prevention and management of chronic GVHD symptoms, including pruritus, rash, pain, dyspigmentation, limited range of motion, erosions, ulcerations, and superinfections (Table 1).
Sun protection is important to reduce the risk of ultraviolet radiation causing an exacerbation in skin chronic GVHD symptoms as well as risk of secondary skin cancers.43 Photoprotection measures include sun avoidance, protective clothing, physical sun blocks, and high-potency sunscreens that protect against both ultraviolet A (UVA) and ultraviolet B (UVB) radiation. Routine lubrication of dry skin with emollients may decrease pruritus and enhance skin integrity.42 Topical steroids and emollients can be used to treat nonsclerotic skin lesions without erosions or ulcerations. Mid-strength topical steroids (eg, triamcinolone 0.1% cream or ointment) can be used to treat skin areas from the neck down. Higher-potency topical steroids such as clobetasol 0.5% can be used in skin areas that are refractory to mid-strength topical treatment. In unresponsive cases, short-term occlusion of mid-strength steroids with damp towels (“wet wraps”) increases skin hydration and steroid penetration.30,41,42 Lower-potency topical corticosteroids such as topical hydrocortisone 1.0% to 2.0% are preferred for long-term use on the face, axillae, and groin. Other adjuvant treatments that can be particularly helpful for pruritus related to GVHD include topical hydrocortisone/pramoxine or menthol-based cream/lotions, as well as systemic antihistamines or the tricyclic agent doxepin.30,41,42 Other interventions such as topical calcineurin inhibitors have been reported to improve erythema and pruritus. For patients with ulcerated skin lesions, wound dressings maintain a moist environment that enhances repair of the epithelium. Protective films can also be useful to prevent breakdown of compromised but nonulcerated skins. Topical antimicrobials may also have some utility to prevent recurrent infections. For patients with major wounds related to skin GVHD, multidisciplinary expertise from dermatology, plastic surgery, and wound care is often needed.
Patients with sclerosis affecting the skin and subcutaneous tissue, including fascia, joints, and the musculoskeletal system, struggle with substantial functional impairments depending on the severity of the sclerosis.30,41,42 Sclerotic features are one of the most frequent and most difficult manifestations of chronic GVHD. Often patients with sclerotic skin GVHD require prolonged treatment with systemic therapies, including corticosteroids, which further causes muscular atrophy, osteopenia, and functional limitations. Table 1 highlights supportive care interventions to address the needs of patients with sclerotic chronic GVHD of the skin.30,41,42
Oral GVHD
Oral GVHD involving the mouth and oral mucosa has 3 components: mucosal involvement, salivary gland involvement, and sclerotic involvement of the mouth and surrounding tissues.30,41,42 Oral chronic GVHD can result in dryness, pain, odynophagia, and taste impairment. New oral lesions should also be evaluated for secondary cancers given that they are more common in allogeneic HCT recipients. Table 2 summarizes management strategies for oral chronic GVHD.30,41,42 Treatment of oral cavity chronic GHVD is mainly focused on using corticosteroid rinses to reduce inflammation in the oral cavity. Dexamethasone or other corticosteroid rinse formulations are held and swished in the mouth for approximately 5 minutes as often as 4 to 6 times per day. For patients with severe symptoms, topical analgesia with viscous lidocaine can also be helpful. Tacrolimus rinses are also an alternative that can be effective.30,41,42 Tacrolimus ointment is especially useful for treatment of lip GVHD involvement. Intralesional injections with triamcinolone can also be helpful for discrete oral lesions that fail to respond to topical therapy.
Patients with chronic GVHD affecting the salivary gland often report dry mouth and sensitivities to hot, cold, spicy, and acidic foods. Patients may also develop mucoceles, which often present as painless blisters on the palate.30,41,42 Supportive care measures for dry mouth include frequent liquid intake, use of salivary stimulants such as sugar-free gum, oral moisturizing agents, and saliva substitutes. Mouth dryness can increase the risk of caries and tooth decay, which can be prevented with topical fluoride use. In patients with severe symptoms, treatment with cholinergic agonists such as cevimeline or pilocarpine may enhance salivary secretions.30,41,42
Ocular GVHD
Ocular GVHD may present with acute conjunctival inflammation, pseudomembranous conjunctivitis, or keratoconjunctivitis sicca syndrome (or dry eye syndrome). Keratoconjunctivitis sicca syndrome is the most common presentation of chronic ocular GVHD and is diagnosed by the presence of symptoms, tear production averaging ≤5 mm (Schirmer's test), and clinical signs of keratitis.30,41,42 Most treatments of ocular GVHD are aimed at relieving dry eye symptoms, including burning, irritation, pain, foreign body sensation, blurred vision, photophobia, and excessive tearing. Table 3 summarizes the recommendations for supportive care management of ocular GVHD.
Preservative-free artificial tears are often used in patients with chronic GVHD to increase lubrication and reduce ocular symptoms.30,41,42 Oral medications can also be used to increase lubrication by stimulating aqueous tear flow with selective muscarinic agonists such as cevimeline or pilocarpine. Management of ocular GVHD also depends on decreasing evaporation.30,41,42 Warm compressors and lid care can maximize meibomian gland output that produces the oil layer of the tear film, which protects against evaporation. Certain protective eye wear such as moisture chamber googles may also help decrease evaporation.30,41,42 Doxycycline is often prescribed for patients with ocular GVHD to treat rosacea blepharitis, thereby reducing the inflammation of the lid. Severe cases may also benefit from scleral lenses, but these are available only in a few specialized centers. To decrease drainage from the ocular surfaces, temporary and permanent occlusions of the tear ducts may help patients with moderate to severe ocular disease.30,41,42 In addition to these measures, patients with ocular surface inflammation may benefit from the judicious use of topical steroid therapy to reduce inflammation. Topical cyclosporine is also commonly used. Autologous tears can also reduce ocular surface inflammation, but these are often available only in specialized centers.30,41,42
Vulvar and vaginal GVHD
Vulvar and vaginal chronic GVHD presents with mucosal abnormalities that can subsequently evolve into sclerotic changes.30,41,42 Patients with vulvovaginal chronic GVHD present with symptoms of vaginal dryness, dyspareunia, and dysuria. If left untreated, vulvovaginal chronic GVHD can result in sclerosis of the vulvar and vaginal tissues, resulting in changes such as agglutination of the clitoral hood, narrowing of the introitus, and shortening of the vaginal canal. Table 4 summarizes the supportive care and management strategies for patients with vulvovaginal GVHD.30,41,42
Itching and irritation can be relieved by the application of emollients to the external genitalia. Water-based lubricants can also be used in the vagina to reduce itching and irritation. Patients with vulvovaginal GVHD often also have low estradiol levels and vaginal atrophy.30,41,42 Topical estrogen therapy with or without the use of vaginal dilators can be extremely effective in the absence of absolute contraindications. For treatment of vulvovaginal GVHD symptoms, topical treatment with ultra-high-potency corticosteroid treatment represents the main therapy, although topical calcineurin ointments can be used in this population as well.30,41,42
Late effects and infection prophylaxis and vaccinations for patients with chronic GVHD
Patients with chronic GVHD are at high risk of late effects, including skeletal complications, secondary cancers, cardiovascular disease, and thromboembolic events.44,45 Due to both chronic GVHD and its treatment, patients often develop metabolic complications, including hypertension, hyperlipidemia, diabetes, and metabolic syndrome.44,45 These, in turn, increase the risk of cardiovascular events. Careful attention to cardiovascular risk factors and these complications is necessary in providing high-quality survivorship care for this population.44,45 Patients with chronic GVHD are highly immunocompromised with deficits in macrophage function, immunoglobulin production, and T-cell function.30,41,42 A review of infection prophylaxis and vaccination strategies for patients with chronic GVHD is beyond the scope of this chapter, but these issues are well outlined in comprehensive guidelines for the prevention of opportunistic infections following HCT published by the Centers for Disease Control and Prevention, the Infectious Diseases Society of America, and the American Society for Blood and Marrow Transplantation.46 Table 5 outlines strategies for monitoring and management of other chronic GVHD complications, including pulmonary GVHD.
CLINICAL CASE (continued)
Despite her gratitude that she is still alive and able to spend quality time with her children, Emma often mourns the immense losses she has experienced as a result of her illness and its impact on her day-to-day life. She also struggles with the uncertainty regarding her future health and prognosis. She is grateful for her transplant clinicians who often validate her emotional struggles, allow her the space to discuss her emotional journey with this illness, and provide supportive resources, including psychosocial counseling to process this difficult illness.
Summary
Living with GVHD affects multiple domains of patient-reported QOL, physical functioning, and psychological well-being. Patients describe living with GVHD as a life-altering “full-time job” requiring unique knowledge, personal growth, and resilient coping strategies. Management of GVHD must include optimal supportive care measures to address GVHD symptoms, promote effective coping, and reduce psychological and existential distress in a population living with immense prognostic uncertainty and struggling to adapt to a difficult and unpredictable illness.
Conflict-of-interest disclosure
Areej El-Jawahri: no competing financial interests to declare.
Off-label drug use
Areej El-Jawahri: nothing to disclose.