Abstract
Children, adolescents, and young adults receiving intensive chemotherapy for acute myeloid leukemia or high-risk or relapsed acute lymphoblastic leukemia sustain prolonged periods of neutropenia that predispose them to invasive fungal disease (IFD). For many decades the standard of care for these patients was to initiate empirical antifungal therapy after a period of prolonged fever and neutropenia. Recent publications have yielded important evidence on the utility of different diagnostic and therapeutic approaches aimed at reducing the impact of IFD among these patients during these vulnerable periods. This case-based review highlights and interprets the published data to provide context for the IFD diagnostic and therapeutic recommendations proposed in multiple published guidelines. Personalized approaches are offered at points where evidence is lacking. Time points where specific knowledge gaps exist are identified along the clinical trajectory of the prolonged neutropenic period to illustrate areas for future investigation.
Learning Objectives
Learn the evidence supporting prophylaxis, preemptive, and empirical antifungal therapy during periods of prolonged neutropenia secondary to intensive chemotherapy for leukemia
Understand the utility and limitations of existing fungal biomarkers at specific clinical time points during prolonged periods of neutropenia
CLINICAL CASE
An 11-year-old girl with recently diagnosed acute myeloid leukemia (AML) is admitted to the hospital for induction 2 chemotherapy. She receives cyarabine, daunorubicin, etoposide, and gemtuzumab ozogamicin in induction 1 and is scheduled to receive cytarabine, daunorubicin, and etoposide for induction 2. What prophylactic antifungal regimen and fungal surveillance testing should be employed once she develops neutropenia? Approximately 2 weeks into her neutropenic period (absolute neutrophil count <200 cells/µL), she develops fevers without any localizing signs or symptoms. Blood cultures are drawn, and cefepime is initiated for empirical antibiotic therapy. The blood cultures are negative and she remains stable, but fevers persist for more than 96 hours. What adjustments should be made to her antifungal regimen, if any, and what additional diagnostic testing, if any, should be performed?
Introduction
Invasive fungal diseases (IFDs) have long been identified as important opportunistic infections in children, adolescents, and young adults receiving chemotherapy for AML and for high-risk or relapsed acute lymphoblastic leukemia (ALL). The epidemiology of IFDs in these patient populations is displayed in Table 1. The interpretation of IFD incidence across each study needs to consider the IFD definition used, whether antifungal prophylaxis was administered, and the study design. Early reports from pediatric AML chemotherapy clinical trials estimated an IFD incidence of 14% to 23% per cycle of chemotherapy.1 More recent investigations have found a lower but still substantial incidence of IFD, ranging from 3% to 7%.2,3 The decline in IFD rates among patients with AML is likely multifactorial, including the use of published research criteria for defining probable or proven IFDs that are more restrictive,3 the decreased use of steroids in this population, and the optimization of supportive care measures. The rates of IFD in ALL are less well defined, but available data suggest the substantial presence of IFD. Initial observational studies across all types of pediatric ALL reported an IFD rate of 10%, with IFD rates nearing 20% in relapsed ALL patients.4,5 However, these studies did not employ the published proven or probable IFD criteria and thus may have overestimated the true rate of IFD. Contemporary studies suggest that proven or probable IFD rates in standard-risk ALL patients have declined to 5.8% but remain high at 12.9% for relapsed ALL patients and at 8.7% to 15.2% for high-risk ALL patients.6,7
Although rates of IFD have varied by time period and by leukemia type, their persistence as opportunistic infections in these patient populations remains a concern. IFDs continue to be a feared complication of leukemia treatment because of the potential for significant morbidity and mortality associated with these pathogens. Invasive candidiasis is the most common form of proven or probable IFD and has been associated with a 10% attributable mortality in children.8 Proven or probable invasive mold diseases, such as aspergillosis and mucormycosis, are less common but have estimated case fatality rates in excess of 30%.9,10
Multiple factors predispose children with leukemia to IFD. They include neutropenia, exposure to high-dose steroids, hyperglycemia, and prolonged broad-spectrum antibiotics.11 Prolonged neutropenia is by far the most documented of these factors. The duration of neutropenia at which the risk for IFD increases significantly is not clearly defined and likely varies by patient. However, in general the risk for IFD is estimated to increase substantially in patients with neutropenia lasting for more than 10 days. The duration of neutropenia will vary depending on the chemotherapy regimen administered, but most contemporary chemotherapy regimens for AML and high-risk or relapsed ALL will result in multiple episodes of sustained absolute neutrophil counts below 200 cells/µL that last for 2 to 3 weeks.11,12 While neutropenia is the dominant risk factor for IFD, the importance of recent steroid exposure in escalating the risk of IFD in leukemia patients should not be overlooked.13,14 The declining reliance on steroids in contemporary AML chemotherapy regimens and increasing reliance on dexamethasone in certain ALL regimens might explain some of the reported decline in IFD incidence in the former and increase in IFD incidence in the latter.15,16
Results from a small randomized trial performed by Pizzo et al in the early 1980s supported the utility of empirical antifungal therapy in patients with persistent fever and neutropenia despite broad-spectrum empirical antibiotic therapy.17 This approach quickly became the standard of care and has remained as such for the better part of 4 decades. In recent years there has been increasing focus on advancing best practices for reducing IFD morbidity and mortality in pediatric AML and ALL patients. Several important investigations and international guidelines have been published that provide guidance on the utility of prophylactic and preemptive antifungal therapy approaches as well as fungal diagnostic tools for these patient populations. In this review we use the provided clinical case to illustrate how published evidence can support important IFD management decisions at various time points during a period of prolonged neutropenia after leukemia chemotherapy. Knowledge gaps in need of future investigation are noted.
Should antifungal prophylaxis be administered during prolonged neutropenia periods?
In the first decade of the current century, the approach to antifungal prophylaxis for neutropenia after receipt of intensive chemotherapy for leukemia varied significantly. An international survey performed by Sung et al found that 77% and 91% of Children's Oncology Group Centers and Berlin-Frankfurt-Muenster Group Centers, respectively, were using 5 different antifungal agents to administer antifungal prophylaxis to children with AML.18 This variation in practice was not particularly surprising because of the limited data from pediatric observational or randomized studies comparing the effectiveness of different antifungal prophylaxis approaches. Adult neutropenia management guidelines did exist at that time and recommended antifungal prophylaxis during periods of neutropenia resulting from intensive chemotherapy. This included a specific recommendation of posaconazole prophylaxis for patients older than 13 years receiving chemotherapy for AML, which was based on randomized controlled trial data revealing the efficacy of posaconazole compared to fluconazole or itraconazole in an adult AML population.19 Fortunately, larger pediatric comparative effectiveness studies soon followed. A retrospective observational cohort study leveraged inpatient hospital administrative data and variation in antifungal prophylaxis utilization to reveal that antifungal prophylaxis in children, adolescents, and young adults with AML did reduce mortality and resource utilization compared to no prophylaxis.20 This study confirmed the presumed benefit of antifungal prophylaxis in pediatric patients but could not determine whether the prophylactic agent needed to include antimold activity. A subsequent randomized controlled trial performed within the Children's Oncology Group (ACCL0933) helped to answer this question by comparing the efficacy of caspofungin prophylaxis with fluconazole prophylaxis.2 In this trial caspofungin prophylaxis significantly lowered the risk of proven or probable IFD, specifically reducing the frequency of invasive aspergillosis.
As these and other pediatric studies began to populate the literature, an international supportive care committee endeavored to collate and interpret the data in a pediatric-specific, systemic antifungal prophylaxis guideline.21 This guideline included a systematic review of studies comparing different antifungal prophylaxis regimens. These data informed a strong recommendation for antimold prophylaxis in pediatric AML patients and a weak recommendation for antimold prophylaxis after intensive chemotherapy for high-risk or relapsed ALL. The latter was a weak recommendation because of limited IFD epidemiology and comparative effectiveness data specific to this population. This weak recommendation coupled with challenges in administering antifungal prophylaxis to children with ALL (eg, greater potential for drug-drug interactions and management mostly in the outpatient setting) has likely resulted in less routine use of antifungal prophylaxis in patients with ALL compared to those with AML. This could, in part, explain the previously cited increasing rates of IFD in ALL patients. Randomized controlled trials assessing the ideal prophylaxis approach in ALL-specific patient populations are warranted.
Notably, the guideline remained flexible on which antimold agent to use for prophylaxis in either AML or ALL patients, suggesting an echinocandin or an azole with antimold activity. The choice of antifungal agent depends on factors such as the age of the child, inpatient vs outpatient setting of neutropenia management, and concern for drug-drug interactions. The guideline did recommend against using a systemic amphotericin formulation for prophylaxis because of a documented increased risk of adverse effects.
Before adapting guideline recommendations to their patients, clinicians need to assess the epidemiology of IFD across their local leukemia population. A center could have differing rates of proven or probable IFD and/or a different fungal pathogen distribution that undermines the generalizability of results from published studies to their patient population. In this setting, a multidisciplinary quality improvement initiative inclusive of oncology, infectious diseases, and antimicrobial stewardship experts could be helpful to guide decisions on local fungal prevention efforts.
My current personal preference is to use an echinocandin as the antifungal prophylaxis agent. This is because echinocandins have a relatively limited side effect profile with less concern about altering the pharmacokinetics of other administered medications. Additionally, our patients with anticipated prolonged neutropenia after intensive chemotherapy are often monitored in the hospital for their periods of neutropenia, so a once-a-day intravenous medication is feasible. Finally, challenges in achieving the appropriate antimold azole dose for each child is a limiting component of this class of antifungal agents. However, as outpatient observation of children during periods of prolonged neutropenia is increasingly utilized, the benefits of enteral prophylaxis will increase, which may favor antimold azoles. Knowledge gaps still exist that if filled would improve the ability to utilize azoles in this setting. These gaps include ideal dosing for children, specifically for posaconazole, and target troughs for prophylaxis.
Is surveillance testing with fungal biomarkers warranted during prolonged neutropenia?
An increasing number of commercially available non-culture-based fungal biomarkers offer the potential for surveillance testing to detect the presence of an IFD at early onset. The most well studied of these biomarkers are the Aspergillus galactomannan (GM) enzyme immunoassay (EIA; Platelia™) and the beta-D-glucan (BDG) assay (Fungitell®). The GM EIA is designed to detect GM, which is a cell wall component of Aspergillus species, while the BDG assay aims to detect BDGs found in the cell walls of various pathogenic fungi, including Aspergillus and Candida spp. Results from studies assessing the utility of these 2 biomarkers as a surveillance tool for early IFD during periods of prolonged neutropenia in adults were received with much optimism. Maertens et al assessed the GM EIA's ability to detect invasive aspergillosis during 362 prolonged neutropenic periods in adults receiving chemotherapy for hematologic malignancy or conditioning for a hematopoietic cell transplantation (HCT).22 They found the operating characteristics of the GM EIA for surveillance testing to be reasonable (sensitivity, specificity, and positive predictive values (PPV) and negative predictive values (NPV) were 72.9%, 99.1%, 93.1%, and 70.8%, respectively). In a similarly designed study, Odabasi et al assessed the BDG assay as a surveillance tool for proven or probable IFD in adults with neutropenia after AML chemotherapy. They found the BDG assay operating characteristics to also be reasonable (sensitivity, specificity, PPV, and NPV were 100%, 90%, 43%, and 100%, respectively).23 These 2 prospective observational cohorts led to US Food and Drug Administration approval for these biomarkers, and many pediatric and adult centers adopted an IFD surveillance testing approach (ie, weekly or twice-weekly GM EIA and BDG assays) during periods of prolonged neutropenia but prior to the onset of any signs or symptoms of IFD.
The transportability of these adult data to children was appropriately questioned. First, an assessment of the BDG assay in otherwise healthy children suggested higher baseline levels of BDG in children and adolescents, which could lead to frequent false-positive results.24 Subsequently, a series of smaller studies were published assessing the GM EIA and the BDG assay in pediatric patients with prolonged neutropenia after chemotherapy or HCT conditioning regimens. These studies were systematically reviewed by Lehrnbecher et al,25 and the results are summarized in Table 2. The operating characteristics varied widely for both assays across studies included in the review. The authors concluded that lower rates (ie, lower pretest probability) of proven or probable IFD in the more recent cohorts increased the likelihood of false-positive results, which limited the utility of surveillance testing. A more recent large observational cohort of pediatric AML patients confirmed the limited utility of the GM EIA and the BDG assays for surveillance testing (Table 2).26 Prior to these publications, our center had been employing weekly surveillance testing with the GM EIA. After reviewing the data from these pediatric studies, we have elected to stop surveillance testing.
Other nonculture diagnostic platforms besides the GM EIA and BDG assays may be appropriate for surveillance testing. These would include mold-specific polymerase chain reaction (PCR) assays, specifically for Aspergillus spp. and pathogens of the Mucorales order, and plasma microbial cell-free DNA (cfDNA) sequencing platforms. Assessments of mold-specific PCRs in pediatric cohorts have focused on scenarios in which the patient is displaying clinical symptoms concerning for IFD (ie, prolonged fever and neutropenia) but they have not been assessed under a surveillance protocol.25 A recent investigation reported the experience of monthly cfDNA sequencing for surveillance of IFD in 40 at-risk pediatric patients with cancer or undergoing HCT.27 While a subset of patients had cfDNA sequencing that revealed the presence of a fungal pathogen, it is not clear that surveillance cfDNA testing improved the clinical management of these patients. Additionally, on some of the cfDNA sequencing results multiple pathogens of unclear significance were identified. Future investigations in larger pediatric cohorts are necessary before considering the commitment of health care resources toward surveillance testing with either PCR or cfDNA sequencing diagnostic platforms.
Empirical antifungal therapy
As noted above, Pizzo et al published their landmark randomized trial in 1982. A total of 34 children, adolescent, and young adult patients with persistent fever and neutropenia, despite broad-spectrum antibiotic therapy, were randomized to receive or not receive systemic conventional amphotericin B therapy.17 Those subjects randomized to antifungal therapy were observed to have a faster resolution of fever. This approach was labeled “empirical antifungal therapy for prolonged fever and neutropenia.” Multiple studies assessing the utility of empirical antifungal therapy were subsequently performed, and collectively, these studies confirmed the benefits of empirical antifungal therapy.28 Based on these data, the 2017 update of the international pediatric fever and neutropenia guidelines recommended that patients at high risk for IFD and with fever and neutropenia persisting longer than 96 hours receive empirical antifungal therapy with either an echinocandin or liposomal amphotericin B.29
However, this recommendation existed prior to the recently published recommendation to administer antimold prophylaxis to patients with anticipated prolonged neutropenia after intensive leukemia chemotherapy. This recommendation to administer antimold prophylaxis at the start of neutropenia raises the question of whether empirical antifungal therapy is still needed. As the example case illustrates, some pediatric patients will develop prolonged fever and neutropenia despite receiving both prophylactic antimold therapy and empirical broad-spectrum antibiotics at the onset of fever. In the randomized trial of caspofungin vs fluconazole prophylaxis in pediatric AML, many patients developed prolonged fever and neutropenia—even those randomized to the caspofungin treatment arm.2 Unfortunately, there are limited data to guide whether a patient like the one in our clinical case should continue the antimold prophylactic agent started at the beginning of neutropenia or if the prolonged fever and neutropenia should prompt a transition to a different antimold agent to serve as empirical antifungal therapy for the remainder of the neutropenic period. This is an important knowledge gap deserving further investigation.
Until data are available, clinicians will need to discuss locally how they want to approach such patients. Notably, the breakthrough proven or probable IFD rate for subjects receiving caspofungin prophylaxis in the randomized trial was 3.1%,2 which may be too high for some clinicians not to change to a different empirical antimold therapeutic. However, a true expansion in antifungal coverage from prophylactic caspofungin would likely necessitate a transition to a lipid amphotericin B formulation, which can have a significant toxicity risk. Therefore, in this situation I elect to review the history and current clinical state of each patient before deciding whether to broaden beyond the prophylactic antifungal agent. If a patient has no symptoms beyond fever and neutropenia and the patient's medical history and exposure history do not increase my concern for IFD, I will recommend maintaining therapy with the same antimold prophylaxis agent.
Preemptive antifungal therapy for prolonged fever and neutropenia
Preemptive antifungal therapy is a concept garnering increasing interest. Evidence from studies assessing this approach may eventually solve the clinical conundrum of whether a patient with prolonged fever and neutropenia should remain on antifungal prophylaxis vs transitioning to empirical antifungal therapy. A preemptive therapy approach relies on the utilization of results from a clinical examination and from diagnostic studies that inform the decision to alter therapy for a given patient. In 2009 Cordonnier et al compared a preemptive antifungal approach to an empirical one in 293 pediatric and adult patients with prolonged or recurrent fever and neutropenia. The preemptive approach included clinical examinations, GM EIA testing, and radiographic imaging. Any signs or symptoms of IFD from those studies prompted the initiation or broadening of antifungal therapy. Preemptive therapy was noninferior for survival and resulted in a significant reduction in antifungal exposure.30 However, significantly more subjects in the preemptive arm sustained a probable or proven IFD. Santolaya et al performed a similar randomized trial in 149 pediatric patients with persistent fever and neutropenia.31 Patients in the preemptive arm had a significant reduction in antifungal exposure and mortality rates similar to those started on empirical antifungal therapy. The 8th European Conference on Infections in Leukaemia referenced the latter study in stating their grade B recommendation that preemptive therapy may be considered as an alternative approach to empirical therapy in pediatric patients.32 The challenge with a preemptive approach in pediatric patients is that it often relies on fungal biomarkers to guide final decisions. Pediatric-specific data on the utility of these biomarkers to either detect or exclude IFD at the time point of prolonged fever and neutropenia are not readily available. As such it is difficult to rely on these biomarkers for this preemptive vs empirical clinical decision. Hopefully, future investigations will define the utility of existing and novel biomarkers for this clinical scenario.
Summary
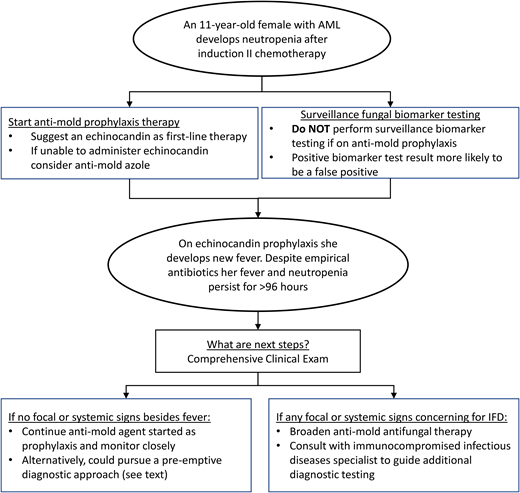
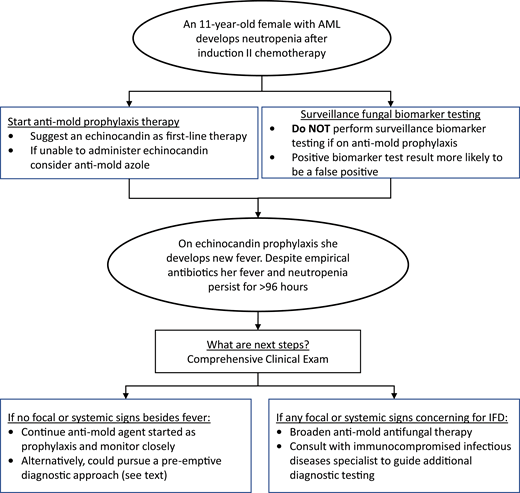
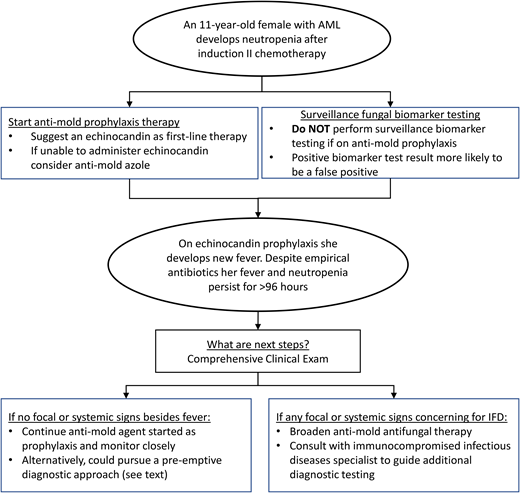
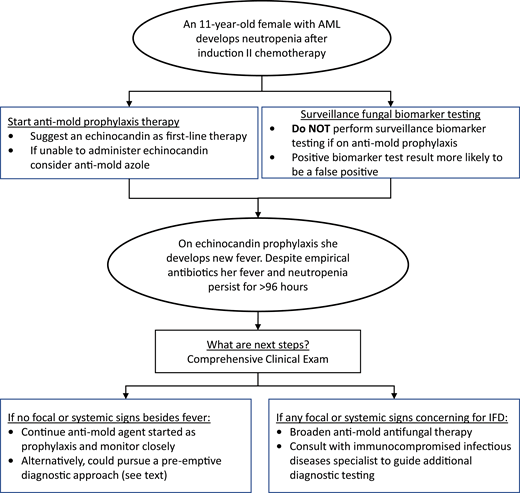
The diagnostic and therapeutic approaches to IFD in pediatric patients with AML and high-risk or relapsed ALL continue to evolve. In the past decade, increasingly available pediatric-specific evidence has informed pediatric-specific guidelines. Figure 1 provides a conceptual model of my interpretation and application of some of the existing data and guidelines detailed above using the proposed clinical case. Antimold therapy for children with anticipated prolonged neutropenia after AML or ALL chemotherapy should be considered the standard of care. While GM EIA or BDG assays should be discouraged for IFD surveillance, more data are needed on using these and other novel biomarkers at other clinical time points. Finally, for patients who develop prolonged fever and neutropenia while on antimold prophylaxis, it is not always necessary to transition to a different empirical antifungal agent. More data are needed to determine the ideal approach to this latter clinical scenario.
Conceptual model for antifungal diagnostic and therapeutic decisions during neutropenia after chemotherapy for AML.
Conceptual model for antifungal diagnostic and therapeutic decisions during neutropenia after chemotherapy for AML.
Conflict-of-interest disclosure
Brian T. Fisher: research funding: Pfizer, Merck; chair: Safety Monitoring Board, Astellas investigation of isavuconazole.
Off-label drug use
Brian T. Fisher: nothing to disclose.