Abstract
Home treatment is feasible and safe in selected patients with acute pulmonary embolism (PE) and is associated with a considerable reduction in health care costs. When establishing a PE outpatient pathway, 2 major decisions must be made. The first one concerns the selection of patients for home treatment. The second one involves dedicated outpatient follow-up including sufficient patient education and facilities for specialized follow-up visits. Current evidence points toward the use of either the Hestia criteria or Pulmonary Embolism Severity Index with/without assessment of the right ventricular function to select patients for home treatment, depending on local preferences. Results from ongoing trials are expected to enforce current guideline recommendations on home treatment and pave the way for more broad application of this elegant and cost-effective management option for patients with acute PE.
Learning Objectives
Get an overview of all published literature on home treatment of acute pulmonary embolism
Understand the evidence based risk stratification tools that can be used to select patients with acute PE for home treatment
Introduction
Acute pulmonary embolism (PE), the most severe presentation of venous thromboembolism (VTE), may be fatal if not diagnosed and treated in time.1 Because of the associated high mortality risk, hospitalization has been the standard of care for all PE patients for monitoring and initiation of anticoagulant therapy. In the past decade, however, studies have shown that PE patients can be stratified into classes of higher or lower risk of adverse outcome based on clinical decision rules, biomarkers, and/or assessment of right ventricular (RV) function.2 Guidelines now recommend formal risk stratification to guide the optimal therapeutic management, and it has been suggested that this may have led to a decrease in PE-related mortality.3,4 This risk stratification cannot only be used to identity patients that benefit from reperfusion therapy but also to select patients who can be managed at home. Indeed, several large studies have been performed showing the safety of home treated PE patients and its benefits with regard to health care costs and patient satisfaction.5-11 Here, we describe the current state of the art of selecting PE patients for home treatment and best practices with regard to PE outpatient pathways.
Case scenario
A 58-year-old woman was evaluated in our hospital because of acute dyspnea and pleuritic chest pain. Symptoms had started 1 week before presentation. She reported no provoking factors for PE nor symptoms suggestive of deep vein thrombosis. Her temperature was 37.2°C, heart rate was 85 beats/min, respiratory rate was 14 breaths/min, oxygen saturation at room air was 98%, and blood pressure was 136/72 mm Hg. Her physical examination and electrocardiogram were unremarkable. The attending physician considered the presence of acute PE. In absence of an alternative explanation, 1 YEARS item was awarded (PE most likely diagnosis), and a d-dimer test was ordered.12 Because the d-dimer level was above the threshold (782 ng/mL; threshold, 500 ng/mL), a computed tomography pulmonary angiography was ordered showing a segmental PE in the left lower lobe. On confirmation of the diagnosis of acute PE, oral anticoagulant therapy was initiated. The attending physician now must decide on the optimal setting of treating this patient: does she require hospitalization or is she a candidate for home treatment?
Home treatment of acute PE
In the literature, outpatient management of acute PE has been referred to as home treatment, early discharge, and outpatient treatment, although a clear definition is lacking. Generally, home treatment is defined as a discharge within 24 hours of initial presentation and early discharge if patients leave the hospital within 3 days. Both home treatment and early discharge involve a much shorter hospitalization than the 7 to 14 days that has been described as the mean admission duration in several European countries.13 In the United States, the median duration of hospital admission for PE was reported to be close to a week.14
There are many benefits of treating patients with acute PE at home. Mostly, patients are saved a hospital admission, which may lead to less anxiety, better quality of life, and higher patient satisfaction. Mostly, however, the health care costs are much lower if (unnecessary) admission is prevented. For instance, it was estimated that at least 25% of patients admitted for PE in the United States could be treated at home. Discharging those patients from the emergency ward would decrease health care costs by an estimated $1 billion each year.15 In the Dutch setting, a recent post hoc analysis of the YEARS study identified a net cost reduction of €1.500 for each patient treated at home.
The severity of the PE and risk of adverse outcomes should largely determine clinical decision making with regard to initial home treatment. Other factors such as locoregional cultural and patient preferences and the structure of the health care system also play an important role. Because of this, major regional differences can be observed. For instance, practice-based studies have shown that 45% to 55% of hemodynamically stable PE patients are treated at home in Canada and the Netherlands, whereas in Spain and France, most patients are hospitalized.13,16-20 The introduction of direct oral anticoagulants with a superior safety profile compared with vitamin K antagonists and many practical advantages have lowered the bar for home treatment of PE.13,21 However, home treatment of PE has not (yet) become the standard of care in 2020. One of the main points of discussion is the threshold of safety (ie, which rate of complications in what time period would be acceptable to treat patients at home rather than in hospital). Although the exact answer to that question is subjective and may vary between individual physicians, patients, and policy makers, one thing is clear. Acute death from hemodynamic deterioration or major bleeding in the first few days after diagnosis is a price too high to pay. Patients at risk for such complications should be hospitalized. Other adverse outcomes such as death from comorbidities (eg, advanced cancer) within the first weeks after diagnosis can, however, not be prevented by hospital admission. Such patients may even prefer being at home surrounded by relatives over hospital admission. Hence, more than strictly adhering to rigid imaging or biomarker thresholds or only focusing on overall mortality, precision medicine is key, tailoring the optimal approach to the individual patient.
Landmark studies
In the last decade, several landmark studies have been published, demonstrating the safety of home treatment in selected low-risk PE patients. These studies are not easily comparable because of heterogeneous selection criteria and various definitions of home treatment. They nonetheless provide important information for the outcomes of home-treated PE patients across a wide range of patient categories and countries.
In the Outpatient Treatment of Pulmonary Embolism study, 344 PE patients (1557 screened for eligibility) were randomized to home treatment or hospitalization.5 First, the Pulmonary Embolism Severity Index (PESI) score was used to identify patients with low mortality risk (Table 1): only patients with PESI class I and II were considered suitable for home treatment. In addition, patients had to fulfill several pragmatic criteria to rule out other factors necessitating hospital admission (ie, being independent from oxygen therapy and having an established support system at home). Patients randomized to home treatment left the hospital of a mean of 0.5 days, whereas patients randomized to hospitalization were discharged after a mean of 3.9 days. Noninferiority was shown in the incidence of recurrent VTE (0.6% vs 0%) and non-PE related death (0.6% vs 0.6%) after a 3-month follow-up period for home treatment and hospitalization, respectively. All patients were treated with a vitamin K antagonist. The incidence of major bleeding exceeded the noninferiority threshold in the home treatment group (1.8% vs 0%). Potential VTE-related medical resource use during follow-up was the same between groups.5
The Hestia study evaluated the efficacy and safety of home treatment in 297 PE patients using the Hestia criteria to identify eligibility for home treatment.6 The Hestia criteria are pragmatic criteria of both risk of mortality and bleeding but also of other reasons for hospitalizing patients with acute PE such as hypoxemia, pain requiring analgesia, and bleeding risk (Table 2). Fifty-eight percent of the PE patients screened for study participation were eligible for home treatment, and 51% were treated at home. The 3-month incidence of recurrent VTE in these latter patients was 2.0% (95% confidence interval [CI], 0.8-4.3), of vitamin K antagonist–associated major bleeding was 0.7% (95% CI, 0.08-2.4), of PE-associated mortality was 0% (95% CI, 0-1.2), and of overall mortality was 1.0% (95% CI, 0.2-2.9).
The VESTA study was a noninferiority trial in which 550 patients with acute PE and none of the Hestia criteria were randomized between immediate home treatment and advanced risk stratification via n-terminal pro-brain natriuretic peptide testing. In the intervention group, patients were treated at home if the NT-proBNP was normal but hospitalized in case of elevated NT-proBNP levels.7 Only 12% of those randomized to NT-proBNP testing had elevated levels and were hospitalized. Noninferiority was shown for the composite outcome of PE- or bleeding-related mortality, cardiopulmonary resuscitation and intensive care unit admission, which occurred in 1.1% (95% CI, 0.2-3.2) and 0% (95% CI, 0-1.3), respectively. The incidence of recurrent VTE was also comparable between the 2 groups: 1.1% (95% CI, 0.2-3.2) for those in the standard of care arm vs 0.73% (95% CI, 0.1-2.6) in the NT-proBNP arm of the study. The most likely explanation for the low number of patients with elevated NT-proBNP is that the Hestia rule preselects patients with normal NT-proBNP levels.7
The eSPEED study was a controlled pragmatic trial designed to evaluate the effect of an integrated electronic clinical decision support system to facilitate risk stratification and decision making at the site of care for patients with acute PE.8 The PESI was used as primary risk stratification tool. After the intervention, the proportion of patients treated at home increased considerably, with a relative increase of 61% (18% preintervention to 28% postintervention), whereas no change was found in the control sites (15% preintervention and 14% postintervention). Importantly, no increases were seen in 5-day return visits related to PE and in 30-day major adverse outcomes associated with clinical decision support system implementation: 12% (95% CI, 5.6-22) vs 6.2% (95% CI, 2.7-12) at the intervention sites vs 9.8% (95% CI, 3.7-20) and 5.1% (95% CI, 1.1-14) at the control sites, respectively.8
In the Low-Risk Pulmonary Embolism Prospective Management Study, 200 patients considered to have low-risk PE based on PESI (class I or II), echocardiography (no signs of right heart strain on echocardiogram), and whole-leg ultrasound of the legs (no proximal deep vein thrombosis) were treated at home with a direct oral anticoagulant.9 Of the 1003 screened patients, 213 were in PESI class I or II and had no other exclusion criteria. Of those, 13 met 1 of the imaging exclusion criteria. The 90-day composite outcome of all-cause mortality, recurrent symptomatic VTE, and major bleeding occurred in 0.5 of patients (95% CI, 0.02-2.4). Patients indicated a high level of satisfaction with their care.9
The most recent study is Home treatment of patients with low-risk pulmonary embolism.10 In total, 525 of 2854 screened patients with acute PE were treated with rivaroxaban and discharged early in the absence of any of the Hestia criteria, signs of RV dysfunction or free-floating thrombi in the right atrium or RV, and contraindications to rivaroxaban. The median length of hospitalization was 34 hours, and 12% of patients were discharged directly on confirmation of the PE diagnosis. The primary efficacy outcome was symptomatic recurrent VTE or PE-related death within 3 months of enrolment, which occurred in 0.6% of patients.10 The incidence of major bleeding was 1.2%, and 2.3% of patients required hospitalization because of (suspected) PE-related complications.
Much more evidence is expected on short notice, notably for the HOME-PE study. In this randomized controlled noninferiority trial, 1975 normotensive PE patients are randomized to risk stratification by either the Hestia rule or the simplified PESI (sPESI) for determining the possibility of home treatment (#NCT02811237). The study will compare the safety and efficacy of both strategies, with the hypothesis that both study groups treated at home because of either none of the Hestia criteria or a low-risk classification by sPESI will have comparable rates of adverse events but that decision making based on the Hestia criteria leads to more patients selected for home treatment.
Best practice for PE outpatient pathways
When establishing a PE outpatient pathway, 2 major decisions must be made. The first one concerns the selection of patients for home treatment. The second one involves dedicated outpatient follow-up including sufficient patient education and facilities for specialized follow-up visits.
According to the literature discussed above, 2 triaging tools have been found adequate for selecting PE patients for home treatment: the Hestia criteria and PESI, with or without biomarker assessment or evaluation of the presence of RV overload. Of note, although the sPESI is much more user friendly than the PESI, well validated, and included in current guidelines, none of the landmark studies on home treatment of PE published to date applied this score.22-24 Even so, it may be assumed that PESI can be substituted with sPESI. For the matter of RV overload, in the Hestia and VESTA studies, RV function evaluation (which is critical to the risk stratification as recommended by the European Society of Cardiology) was not part of standard baseline assessment. As a consequence, 30% of all patients treated at home had a RV/left ventricular (LV) diameter ratio > 1.0, without a higher incidence of adverse outcome: the combined 3-month incidence of recurrent VTE and all-cause death was 2.7% in patients treated at home with a RV/LV diameter ratio > 1.0 and 2.3% in patients with a normal RV/LV ratio.25 Furthermore, high sensitive troponin-T (hsTnT) did not have an additional prognostic value on top of Hestia, as was the case for NT-proBNP in the VESTA study.7,26 The adverse 30-day composite outcome of hemodynamic instability, intensive care unit admission, or death related to either PE or major bleeding occurred in 1.7% patients treated at home with post hoc measured elevated hsTnT levels compared with 0.70% with normal hsTnT (odds ratio, 2.5; 95% CI, 0.22-28). All-cause death occurred in 1.7% of patients in both groups (odds ratio, 1.0; 95% CI, 0.11-8.7).26 These observations suggest that the hemodynamic profile of a patient (ie, the severity of RV overload and the resulting hemodynamic response) rather than just an abnormal RV/LV ratio or NT-proBNP is intrinsically taken into account in the decision to treat patients at hospital or at home when applying the Hestia criteria. Hence, in our practice, we use the Hestia criteria without further explicit (imaging) biomarkers. If PESI is used, parameters of the hemodynamic profile of the patients are included in the risk stratification, but RV function is not. Because PESI with/without measures of RV overload focuses on risk of early adverse alone and not on assessing the possibility of home treatment, PESI should always be combined with other Hestia-like criteria for this purpose as was done in the Outpatient Treatment of Pulmonary Embolism study.5
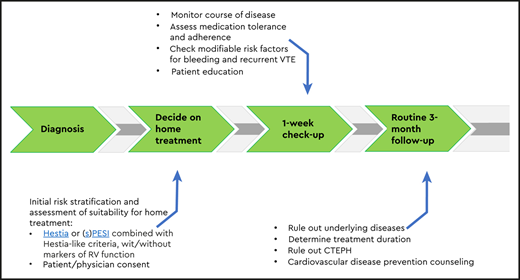
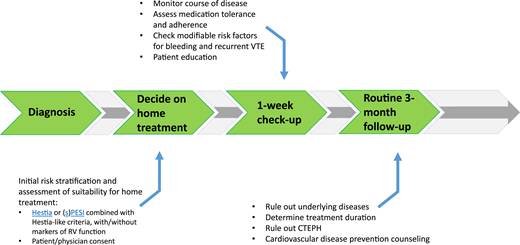
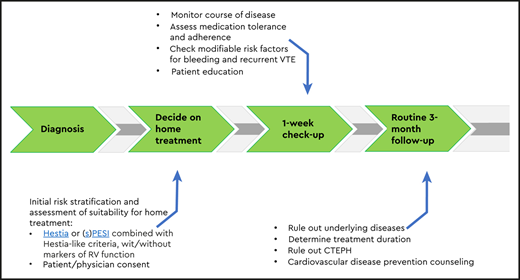
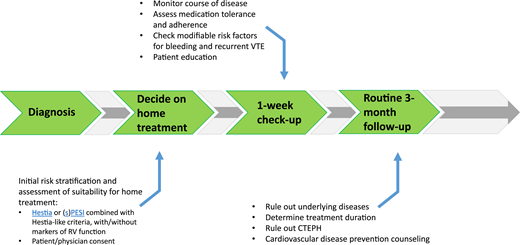
If patients are treated at home, a proper outpatient pathway should be in place (Figure 1). First of all, patients need to receive preferably written instructions on who and when to contact in case of alarm symptoms. Second, in most studies, patients were contacted by telephone or evaluated in an outpatient clinic in the first week after diagnosis. This is a very reasonable approach in practice-based conditions as well. At that moment, it is important to check the vital parameters, as well as whether the patient is doing well, follows the anticoagulant drug prescription, is aware of alarm symptoms, has received sufficient patient education, and has no untreated modifiable risk factors for complications such as major bleeding.27-29 If the patient is recovering according to expectation and if no other interventions are necessary, the routine patient pathway can be followed, with additional visits to establish the optimal duration of anticoagulation and, if indicated, tests to rule out underlying disease. In general, outpatient pathways should be collaborative between general practitioners and thrombosis specialists, including fast exchange of a medical reports and/or discharge letters to all involved.30
Resolution to the case
The patient was hemodynamically stable and required no other treatment than (oral) anticoagulation. None of the Hestia criteria were present, and home treatment was discussed with the patient. She lived together with her husband who could take care of her, and she responded favorable to the suggestion of home treatment. Six days after immediate discharge from the emergency department, she visited our dedicated thrombosis outpatient clinic. A specialized nurse evaluated the initial course of disease, presence of complications, and risk factors for complications (eg, by measuring blood pressure and checking medication adherence). It was concluded that the patient was recovering well, had taken the medication in accordance with the prescription, and was at low risk of complications. Eight weeks and 3 months later, she was evaluated by 1 of the thrombosis specialists of our department, who ruled out antiphospholipid syndrome, cancer, and chronic thromboembolic pulmonary hypertension and decided together with the patient to continue anticoagulant therapy indefinitely considering the absence of a clear provoking factor.
Conclusion
Home treatment is feasible and safe in selected PE patients and is associated with a considerable reduction in health care costs. Current evidence points toward the use of either the Hestia criteria or PESI with/without assessment of the RV function to select patients for home treatment. Results from ongoing trials are expected to enforce current guideline recommendations on home treatment and pave the way for more broad application of this elegant and cost effective management option for patients with acute PE.
References
Competing Interests
Conflict-of interest disclosure: F.A.K. received research grants from Bayer, Bristol-Myers Squibb, Boehringer-Ingelheim, MSD, Daiichi-Sankyo, Actelion, the Dutch Thrombosis Association, and the Dutch Heart Foundation. M.V.H. received research grants from ZonMW, Boehringer Ingelheim Bayer Health Care, and Pfizer-Bristol-Myers Squibb; and received consultancy and lecture fees from Pfizer-Bristol-Myers Squibb, Boehringer Ingelheim, Bayer Health Care, and Aspen.
Author notes
Off-label drug use: None disclosed.
CorrespondenceFrederikus A. Klok, Department of Medicine–Thrombosis and Hemostasis, Leiden University Medical Center, LUMC Room C7-14, Albinusdreef 2, 2300RC, Leiden, the Netherlands; e-mail: f.a.klok@lumc.nl.