Abstract
Consistent with observations in other disease settings, retrospective studies have indicated that treatment outcomes for adults with acute myeloid leukemia (AML) are better in higher- vs lower-volume hospitals and academic vs nonacademic centers, with greatest benefits noted in acute promyelocytic leukemia. Younger age, more frequent receipt of chemotherapy and hematopoietic cell transplantation, and differences in comorbidities and socioeconomic factors may partially account for these differences. With new therapeutic options including oral small molecule inhibitors and parenteral drugs suitable for outpatient administration, there is increasing interest from patients and physicians in treating AML in the community setting and avoiding referral to academic centers. This may be particularly true for older adults, for whom treatment rates in the community have historically been low, and for those with comorbidities, because treatment benefits are estimated to be low, and thus travel to academic centers is perceived as especially burdensome. How the volume-outcome relationship is affected by the shift of the treatment landscape in AML over the last few years is unknown. Additionally, improvements in supportive care (transfusion support, broad-spectrum oral antimicrobials), resulting in gradually decreasing early death rates over time, and the growing focus on the impact of AML therapy on quality of life and treatment cost concerns further fuel the larger trend toward an increasing proportion of care delivered in the outpatient setting. Here, we examine whether the current shift of administering chemotherapy and supportive care to the outpatient setting can be translated to the community setting without compromising patient outcomes.
Learning Objectives
Understand the impact of center type on outcomes in patients with AML
Recognize characteristics of academic centers that may account for differences in outcomes compared with community settings
Consider how emerging diagnostic and monitoring techniques, together with the availability of new drugs, will affect care delivery to AML patients in the future
Introduction
Until recently, treatment options for acute myeloid leukemia (AML) were relatively limited, and decision making followed an algorithm in place for almost 50 years.1,2 For medically fit patients, cure was assumed possible with intensive chemotherapy and possibly allogeneic hematopoietic cell transplantation (HCT). Because of transfusion needs and risks of disease/treatment-related complications, patients receiving intensive therapies typically remained in the hospital, either at academic centers or nonacademic facilities, until resolution of cytopenias. In contrast, if the patient was judged medically unfit, cure was considered rare, prompting either nonintensive chemotherapy, most commonly low-dose cytarabine or single-agent azacitidine or decitabine, or an approach purely focused on supportive care.
Since 2017, the US Food and Drug Administration has approved 8 new drugs for AML in the United States.3 With these, treatment options have substantially increased, and the line dividing intensive and less-intensive therapies has become less clear. With oral small-molecule inhibitors and parenteral drugs suitable for outpatient administration included, and helped by improvements in supportive care with resulting declines in early death rates, interest is mounting from patients and physicians in treating AML in the community. This may be especially true for older adults and/or those with comorbid illnesses, because expectations for treatment benefits may be low; hence, travel to and treatment at academic centers be perceived as particularly burdensome. Treatment rates for such individuals in the community have historically been low. This trend is further fueled by an increasing interest in the impact of AML therapy on quality of life (QOL) and growing concerns over costs. Here, we review the evidence for and against the need to treat AML at academic centers and examine whether the current shift of transitioning therapy and supportive care to the outpatient setting can be translated to the community without compromising patient outcomes.
Volume–outcome relationship in AML
For many medical conditions and surgical procedures, both in oncologic and nononcologic settings, numerous studies and meta-analyses have shown a strong correlation between increasing patient volumes and better outcomes.4,5 A study reported several years ago6 suggested AML is no exception to this. A more recent analysis of patterns of care and clinical outcomes with conventional induction chemotherapy (IC) across diverse practice settings supports this conclusion by showing AML patients treated in high-IC–volume hospitals were less likely to die or be discharged to hospice than those treated at low-IC–volume hospitals.7
Rather than examining patient volume in the strict sense, however, most studies (all retrospective) have compared academic with community centers, with individual studies differing in how to define academic centers (patient volume vs designation as comprehensive cancer center by the National Cancer Institute or academic center by the Commission on Cancer), complicating comparisons and data interpretation. As one example, data from >60 000 AML patients treated in the United States between 2003 and 2011 suggested lower early death risks and better 1- and 5-year overall survival at academic centers.8 The benefit was greatest for patients with acute promyelocytic leukemia (APL), consistent with previous observations.9,10 However, although many studies have suggested better outcomes at higher-volume or academic institutions, findings have not been entirely uniform. In the European Organization for Research and Treatment/Gruppo Italiano Malattie Ematologiche dell’ Adulto AML 8A study, for instance, which included patients treated at both transplant centers and referring centers (analogous to community centers), early death rates were higher and initial remission rates lower at referring centers, but remission rates after the second induction course and 6-year overall survival were similar.11
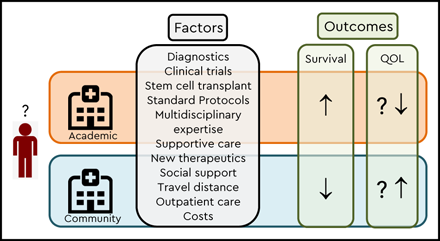
There are many reasons why AML patients may do better with nontransplant therapies at academic (or higher-volume) centers (Table 1). For one, patients treated at academic centers, especially when older, are more likely to receive chemotherapy than their counterparts in the community.12,13 Furthermore, physicians, other medical providers, and supportive and ancillary staff (eg, physical therapists and nutritionists) at academic centers may have more experience managing disease/treatment-related complications of AML and have greater access to multidisciplinary teams and subspecialists dedicated to managing challenging complications The observation of lower early mortality when treated at academic centers8 supports the notion of better supportive care playing a pivotal role in survival differences, with one study showing half the risk of early death at National Cancer Institute–designated cancer centers compared with private hospitals with lower rates of renal failure, respiratory failure, and cardiac arrest14 . Similarly, another study found patients treated at higher-volume hospitals were more likely to undergo bone marrow assessment and receive prophylactic antimicrobials than those treated at lower-volume hospitals.7 Whether better access to clinical trials at academic centers translates into better outcomes (trial effect) remains controversial13,15-17 ; restrictive eligibility criteria for trial participation may bias such analyses.18
Perhaps unsurprisingly, the center effect in AML extends to survival outcomes with allogeneic stem cell transplantation (HCT), which are closely linked to overall AML outcomes.19 Whether such benefits extend to other post-HCT composite endpoints such as graft-versus-host disease–free, relapse-free survival is, although plausible, currently unknown. Underlying reasons may include that patients treated at academic centers more likely undergo allografting, possibly because of earlier HLA typing and donor identification, easier care coordination facilitating the transplant workup and reducing the time to transplant, and access to a broader range of transplant protocols. Moreover, expansion of eligibility for allogeneic HCT (eg, to include larger numbers of older adults) combined with the fact that older patients are more likely to receive antileukemia therapy may contribute to improved overall outcomes at academic centers.
Attempts to decipher whether outcomes are better at higher-volume/academic centers are complicated by the increasing heterogeneity within academic settings as private oncology practices are bought by academic health systems, because this academic affiliation does not necessarily come along with the expertise or supportive care capabilities that long-standing academic centers provide. There are also likely unaccounted-for differences in the characteristics of patients treated at different sites. These confounders could be addressed by multivariate analyses accounting for prognostic covariates and site of treatment, but such models have limited prognostic ability, indicating many important prognostic factors remain unknown. Only randomization between treatment at academic or community centers can account for such latent variables.
Are there distinct patient subsets that derive particular benefit from treatment at higher-volume or academic centers?
Certain subsets of leukemia patients have unique needs and challenges and may particularly benefit from the treatment environment offered at high-volume centers. As mentioned above, this is particularly true for patients with APL when treatment is initiated at academic centers.9 This may be partially reflected in the substantially lower early death rates observed in the context of clinical trials (which are largely conducted at academic centers) compared with those seen in the general APL population,20 although selection bias may play a role as well. Earlier diagnosis, faster availability and initiation of ATRA,21 and improved diagnosis and management of complications (eg, disseminated intravascular coagulation, differentiation syndrome) may be contributing factors. Another distinct subset of patients particularly benefiting from higher-volume/academic centers are adolescents and young adults (AYA). As one example, one study evaluating outcomes of AML patients 18 to 39 years of age reported improved survival specifically in those with good-risk cytogenetics and those with APL when treated at academic centers, with the latter having half the risk of early death compared with patients treated at community centers.10 Similarly, available evidence suggests that AYA with acute lymphoblastic leukemia (ALL) likewise benefit from treatment at academic sites, even after controlling for sociodemographic features.22 Conceivably, this observation is closely linked to better outcomes of AYA with ALL following pediatric-inspired treatment regimens, which, because of their complexity, are more likely to be given at academic sites.23 Increased enrollment on clinical trials of AYA with ALL at academic sites may also be contributing. Although data are lacking, it is likely that the same features that contribute to better outcomes in AYA with ALL and AML in general (better access to diagnostic testing and complex supportive care) may also apply to older adults with ALL given the complexity of the treatment regimens, many of which are now administered in the outpatient setting.
Several studies have evaluated whether improved outcomes seen at academic centers extend to older adults, for whom travel to these centers might be more burdensome and overall treatment outcomes worse. Prospective and registry data suggest that such patients do benefit from both intensive and less-intensive chemotherapy compared with no therapy.24-26 However, intensive induction strategies are very rarely used in the community setting. In fact, most older patients with AML do not receive any type of AML-directed therapy, as indicated by data from a large retrospective Surveillance, Epidemiology, and End Results Medicare study showing only 40% of adults >65 years of age received anti-AML therapy within 3 months of diagnosis.27 Results were similar in an analysis of US community oncology practice data,12 with another Surveillance, Epidemiology, and End Results study showing >50% of AML patients >65 years of age received no anti-AML therapy even 9 years after azanucleosides (eg, azacitidine and decitabine) became available. Patients living in large metropolitan areas (with easier access to academic centers) were more likely to receive treatment, as were patients with a previous diagnosis of a solid or hematologic malignancy despite reduced performance status, possibly because of already having established specialist care.28 Finally, for those who do receive azanucleoside therapy, published dose schedules are often not adhered to in the community (partially related to limited weekend infusion hours), and only a minority of patients surveyed in a recent population-based study in the United States received the recommended ≥4 cycles of therapy, potentially limiting the efficacy of these agents.29
Transition to outpatient delivery of intensive AML-directed therapy and supportive care
Historically, intensive therapy for AML has been delivered in the hospital in both academic and community settings because of the need for frequent transfusions and the likely occurrence of treatment/disease-related complications. However more recently, with improvements in supportive care such as introduction of broad-spectrum oral antifungals, ready availability of high-quality blood products, approval of new antileukemia drugs, and increasing focus on QOL30 and treatment-associated costs, there has been a shift in care patterns with increasing efforts to administer chemotherapy and supportive care in the outpatient setting.
With availability of new drugs such as CPX-351 that have limited immediate toxicities and relatively convenient dosing schedules, there are now intensive treatment options that can be administered in the outpatient clinic even to older patients, both in the community and academic settings. Difficulties in recovering inpatient costs provide an additional incentive, prompting many centers to shift to outpatient administration of CPX-351,31 with pilot data suggesting patients may remain outpatient after therapy,32 although some centers currently routinely admit patients to the hospital for monitoring once CPX-351 is administered. Likewise, although treatment with venetoclax in combination with either low-dose cytarabine or an azanucleoside can be given in the outpatient setting, validated guidelines on how best to administer such therapies are currently missing, and many institutions admit patients routinely at the beginning for close monitoring of potential treatment-related complications (tumor lysis syndrome). Nonetheless, even conventional intensive induction and postremission therapy can be safely delivered in the outpatient setting in many patients.33,34 However, given the complexities surrounding drug administration schedules and management of postchemotherapy care, a multidisciplinary team including social workers, nurses, pharmacists, and providers with expertise in the care of AML (largely available in academic centers only) is critical for successfully implementing this approach.
Despite this interest in administering newer therapeutics in the outpatient setting, most patients are still hospitalized for prolonged periods of time after intensive induction chemotherapy because of disease/treatment-related cytopenias, transfusion needs, and management of related complications.35 This may, however, not be necessary for many patients. Based on multiple small studies suggesting feasibility of outpatient management after conventional induction chemotherapy,36 we conducted 2 prospective clinical trials at our institution evaluating an early hospital discharge (EHD) strategy within 3 days after completion of intensive induction chemotherapy.37,38 These studies supported the notion that early transition to outpatient care is feasible, safe, and associated with reduced care costs, which has since become standard of care at our institution, logistics permitting. We recently evaluated our experience with this approach in the 4-year period since we completed our trials, with the application of the EHD strategy to a much broader patient base than was captured in the prospective trials, with confirmation of safety and reduced medical resource use.39 Of note, we found no significant differences in care needs for patients undergoing initial induction treatment and those receiving postremission therapy.40 This suggests that an EHD care strategy after induction therapy may be practically (and safely) implemented at many institutions that already have the infrastructure available enabling them to manage patients in the outpatient setting after standard postremission chemotherapy. At our institution, infrastructure available to support outpatient management of AML patients during the time of prolonged pancytopenia includes 24-hour phone access for patients to a provider familiar with outpatient management of AML, an infusion center with extended daily hours (including weekends and holidays) for transfusion needs, and the ability to rapidly evaluate and initiate treatment of neutropenic fever in the outpatient clinic before hospital transfer. These features (summarized in Table 2) are more likely present at academic than community cancer centers.
Unlike induction therapy, follow-up care after postremission chemotherapy has already shifted to the outpatient setting at both academic and community centers,35 with multiple studies demonstrating feasibility, safety, and cost-effectiveness. For some patients, receiving outpatient care at the center where the chemotherapy was administered may pose logistic challenges. Here, a shared care model may address this barrier by allowing patients to receive their supportive care after postremission therapy at community centers closer to their homes.41 Finally, the COVID-19 pandemic has forced rapid improvements in technology supporting telehealth, along with reimbursement for this service, potentially allowing academic sites to oversee some aspects of patient care with less travel for patients. How the rapid increase in access to telehealth will play out for the care of leukemia patients over the next few years remains unknown.
What about other major drivers for the shift from inpatient to outpatient care: QOL and health care costs? Studies in patients with hematologic malignancies undergoing autologous or allogeneic HCT have shown hospitalization is associated with reductions in QOL and increased depression.42 The same has been found in AML.30 Although there are no data comparing QOL of AML patients treated at academic vs community centers, more time spent in the outpatient setting (and closer to home) for both treatment and follow-up care may lead to gains in overall QOL. The same might apply to care costs, which remain dominated by inpatient charges.43 The shift to more outpatient care and potentially more community-based care that newer medications facilitate may ultimately offset at least part of their high costs.
Will treatment at a higher-volume/academic center remain important with new lower-intensity and/or oral drugs?
Many of the new drugs in AML are molecularly targeted agents that are given orally and can be used at various stages along the AML treatment path. Whether there is a measurable benefit when such agents are given at a higher-volume/academic center vs the community setting is unclear. Undoubtedly, the availability of effective oral drugs (eg, venetoclax and oral decitabine/azacitidine in the AML pipeline) used alone or along with parenteral lower-intensity therapeutics (eg, azanucleosides) will increase the proportion of patients treated at community centers, including older and less-fit individuals. Thus far, the risk/benefits of delivering lower-intensity therapies at academic vs community centers are unknown (an important research agenda item for the near future), especially because these therapies can still lead to prolonged cytopenias and toxicities; thus, the issue of which centers provide better supportive care will likely remain relevant. In what way a center’s access to molecular testing influences treatment outcomes is also unknown. For some of the newer agents (eg, inhibitors of mutated IDH1/IDH2/FLT3), anti-AML efficacy is primarily seen in patients carrying the corresponding mutations in their leukemia cells. Appropriate use of these therapeutics therefore requires access to timely molecular testing. One could therefore argue better testing availability at academic institutions may ultimately translate into better outcomes for patients getting care at such centers. However, although some data suggest molecular testing is more widely available at academic centers,44 there is no clear evidence yet linking this availability to improved outcomes. It also remains uncertain whether targeted therapy in general will yield longer survival than nonspecific clinical trials.45 Finally, many of these new drugs are costly to patients (particularly the oral drugs that come with high copays) that many, especially older individuals, cannot afford. With their resources, academic centers may be better positioned to help them find financial support from foundations and pharmaceutical companies to obtain these therapies.
Conclusion and future perspective
Increasing access to new drugs has shifted the care of AML patients from academic to community settings. Thus far, this change in care pattern remains unsupported by data, especially for older and less fit patients who historically have not received antileukemia therapy in the community. Some of the potential advantages that academic centers provide (access to more rapid molecular testing, a broader range of clinical trials and allogeneic HCT, greater disease expertise, and availability of multidisciplinary teams for supportive care) have to be weighed against potential advantages of community settings (eg, less disrupted life, better family support, and QOL). With the rapidly changing treatment landscape in AML, the pros and cons of academic vs community setting treatment will need to be revisited constantly, ideally via randomized trial, as challenging as this would be. In the absence of strong data arguing for/against a particular care scenario, a shared academic-community care approach may currently best serve the interests of many patients.
Acknowledgments
The authors thank Elihu H. Estey, Charles A. Schiffer, and Wendy Stock for critical reading of the manuscript.
References
Competing Interests
Conflict-of interest disclosure: The authors declare no competing financial interests.
Author notes
Off-label drug use: None disclosed.
CorrespondenceAnna B. Halpern, 825 Eastlake Ave E, Box CE3-300, Seattle, WA 98109-1024; e-mail: halpern2@uw.edu.