Abstract
Classic Hodgkin lymphoma (cHL) stands out as success story in the field of medical oncology, with multiagent chemotherapy with or without radiation leading to durable remission for most patients. Large-scale clinical trials during the past 40 years have sought to minimize toxicities while maintaining strong efficacy, including efforts to reduce the size of radiation fields, minimize alkylator chemotherapy, reduce the number of chemotherapy cycles, and omit radiation in select populations. The last decade has also ushered in novel therapies, including brentuximab vedotin (BV), that have improved clinical outcomes for patients with cHL resistant to standard cytotoxic therapies. More recently, a large randomized trial compared BV plus chemotherapy with chemotherapy alone for first-line treatment of advanced stage cHL. With ∼24 months of available follow-up, the BV containing regimen was found to be associated with a reduction in the risk of progression, death, or incomplete response to first-line treatment (modified progression-free survival). Whether this early signal of improved efficacy is worth the additional acute toxicities and added drug-related expenses associated with incorporating BV into first-line treatment remains controversial. This chapter provides historical background; reviews the cost-effectiveness of available cHL therapies; and summarizes potential ways to balance innovation, affordability, and patient access to novel therapeutics.
Learning Objectives
Use a case of a patient with Hodgkin lymphoma as an exemplar to review cost-effectiveness analyses and budgetary impact in modern oncology
Discuss potential approaches that balance innovation, value, and access to novel cancer therapies
Clinical case
A 36-year-old woman presented to her primary care provider with fatigue and generalized pruritus progressive over 6 weeks. Her examination was notable for palpable lymphadenopathy up to 2.5 cm in the bilateral neck. She was referred for an excisional lymph node biopsy, which confirmed involvement by classic Hodgkin lymphoma (cHL), nodular sclerosing subtype. Staging positron emission tomography (PET)/computed tomography (CT) was consistent with stage IV disease, with fluorodeoxyglucose-avid lung nodules, splenic lesions, and abnormal uptake in lymph nodes above and below the diagram. The patient was a nonsmoker, and her echocardiogram and pulmonary function tests confirmed preserved baseline organ function. Other than leukocytosis (white blood cell count of 16 × 109/L), results of her blood work were normal, and her international prognostic score (IPS; Hasenclever Index) was calculated at 2 (stage IV, white blood cell count ≥15 × 109/L). Her social history was notable for having 2 children and not having any desire for additional offspring. She was insured through a US employer–sponsored high-deductible health plan with maximum annual out-of-pocket expenses of $10 000.
Standard treatment with cytotoxic chemotherapy
Modern medical oncology as a specialty can trace its humble beginnings to the perseverance of “chemotherapists” from the 1960s, who combined available cytotoxic drugs to develop successful multiagent regimens for patients with advanced cHL.1 Since initial efficacy was established, considerable clinical efforts over the ensuing decades have focused on minimizing toxicities and improving efficacy. Important advancements in the first-line setting include the development of current standard chemotherapy regimens (ABVD [doxorubicin, bleomycin, vinblastine, dacarbazine] and escalated BEACOPP [bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone]),2,3 recognition of long-term toxicity related to radiation therapy,4 and the development of approaches to ameliorate these risks.5 More recently, large-scale clinical efforts have used interim PET to tailor therapeutic strategies on the basis of the depth of early treatment responses.6 These modern risk-adapted approaches are successful for the majority of patients with cHL.5,6 Patients with nonbulky limited stage disease have particularly good outcomes after first-line therapy, with recent studies establishing that abbreviated courses of ABVD plus consolidative radiation offer durable remissions in >90% of individuals.5,7-9
More controversy surrounds the selection of multiagent chemotherapy for individuals with advanced stage cHL. In the United States, 6 cycles of ABVD has generally been viewed as the standard therapy for most patients with stage III/IV cHL. This approach is predicated on the recognition that escalated BEACOPP offers an improvement in progression-free survival (PFS) over ABVD,10 but these gains are not believed to outweigh the added toxicities for most patients. Consensus guidelines in the United States acknowledge escalated BEACOPP as being “useful in certain circumstances,” such as in young patients (ie, <60 years old) with high-risk disease (IPS 4+).11 Conversely, escalated BEACOPP (4-6 cycles) remains a standard therapy for advanced stage cHL in some European nations, with European Society for Medical Oncology guidelines including it as a suitable treatment in those <60 years of age, regardless of IPS score.12
Although salvage chemotherapy followed by autologous stem cell transplant (ASCT) in responding patients remains a standard therapy for relapsed or refractory (R/R) cHL, responses and durability of chemotherapy are less satisfactory than in the first-line setting. Outcomes with salvage chemotherapy and ASCT remain relatively favorable in younger patients relapsing >12 months after completing primary therapy.13 However, patients with primary refractory disease or relapse within 12 months of completing first-line treatment are unlikely to derive durable remissions with this chemotherapy-only approach. Furthermore, outcomes of patients with cHL relapsing after autologous transplant are poor, with historical cohorts having a median overall survival of just 29 months.14
Novel therapies for the treatment of Hodgkin lymphoma
Early efforts to develop novel cHL therapies appropriately focused on the critical need to improve outcomes for patients with cHL refractory to cytotoxic chemotherapy. The CD30 antibody–drug conjugate brentuximab vedotin (BV) became the first approved therapy for this setting in 2011 on the basis of a promising phase 2 study. In that study, 102 patients with R/R cHL progressing after ASCT were treated with single-agent BV for up to 18 cycles.15 Patients had a favorable objective response rate of 75%, including 35% with complete remission.15 Importantly, updated results with 4+ years of follow-up showed a median survival of 40.5 months, with some patients remaining in remission without the need for additional cHL-directed therapy beyond single-agent BV.16 The efficacy of single-agent BV has since been confirmed in a double-blind randomized trial comparing BV with placebo in the post–autologous transplant consolidation setting.17 Use of post-transplant consolidative BV in high-risk patients reduced the risk of progression by ∼40%.17
Immunotherapy with programmed cell death protein 1 (PD1) blockade is another highly efficacious therapy for patients with chemorefractory cHL. The anti-PD1 monoclonal antibodies pembrolizumab and nivolumab have both been tested in single-arm phase 2 studies in patients with R/R cHL.18,19 Available data suggest similar overall response rates between the 2 agents (65%-70%), with ∼30% of patients achieving complete remission.18,19 Durability of response remains less certain, but a recent update with a median follow-up of 18 months found that nivolumab provided a median PFS of 14.7 months and a median duration of response of 16.6 months.20
The clear efficacy of BV and anti-PD1 inhibitors seen in the previously challenging clinical setting of chemorefractory cHL allowed singe-arm phase 2 trials to lead to their accelerated approval and rapid clinical adoption in the United States. Conversely, the well-established and favorable efficacy of combination chemotherapies for first-line cHL clearly requires well-designed randomized trials before novel therapies are embraced in this setting. Such a trial was recently reported; it compared BV + AVD (brentuximab vedotin, doxorubicin, vinblastine, dacarbazine) with standard ABVD in patients with stage III/IV cHL.21 After a median follow-up of 24.6 months, BV + AVD was associated with a 23% reduction in the risk of progression, death, or incomplete response to first-line therapy leading to subsequent anticancer treatment (modified PFS).21 Acute toxicities were also different between the treatment arms, with febrile neutropenia and overall grade 3+ toxicities more common with BV + AVD but severe pulmonary toxicities more likely with ABVD (7% vs 3%). Ultimately, the confirmation of a modified PFS benefit with ∼2 years of follow-up led regulatory bodies in the United States, Europe, and Japan to approve the marketing of BV in combination with AVD for the first-line setting.
Cost of available first-line cHL therapies
Together with clear differences in acute toxicities between BV + AVD compared with ABVD, combining BV with chemotherapy adds significant drug-related expenses. Dosed every 14 days at 1.2 mg/kg and available only in 50-mg single-use vials, BV is associated with greater drug-related costs than ABVD. Transparency of drug pricing is lacking, and reimbursement varies significantly across payers and countries. However, for the sake of comparison, the average US sales prices (April 2019)22 for first-line regimens are displayed in Table 1. These average sales prices reflect the weighted averages of all manufacturers’ sales prices and include all rebates and discounts that are privately negotiated between manufacturers and purchasers in the United States. It can be seen that drug expenses in the United States are >100 times higher for BV + AVD than for ABVD ($33 834 vs $277 per 28-day cycle).
Cost-effectiveness of BV + AVD vs ABVD in the first-line setting
Although BV is associated with greater drug expenses than ABVD, the fact that fewer patients relapse after BV + AVD has the potential to reduce downstream health care expenditures and improve important clinical outcomes (ie, quality and quantity of life) compared with standard ABVD. As a form of economic analysis, cost-effectiveness analyses (CEAs) go beyond consideration of initial health care expenditures and allow direct comparison of relative costs and outcomes among different health decisions over extended time horizons. These analyses aim to calculate the average trade-offs between therapeutic strategies and are increasingly viewed as a key tool for optimizing health care resources.23,24 Although recent guidelines for CEA hold great promise in improving consistency and comparability across studies,25 a degree of subjectivity remains when building CEA models.
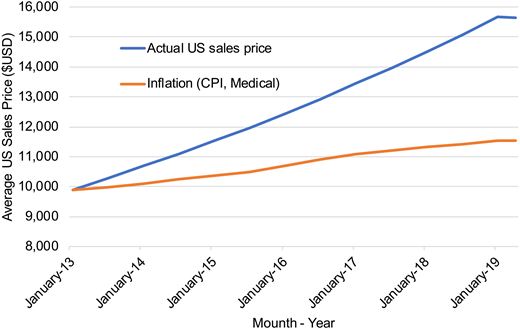
Two separate groups have published CEAs for BV + AVD compared with ABVD. Huntington et al26 estimated BV + AVD to be associated with improvements in both life-years gained and quality of life, with their base case model estimating a 0.56 quality-adjusted life-year (QALY) improvement for BV + AVD compared with ABVD. However, health care costs associated with BV + AVD compared with ABVD were considerable at $176 846 in 2017 US dollars.26 As a result, the authors estimated the incremental cost-effectiveness ratio (ICER) for BV + AVD in the first-line setting to be $317 254/QALY compared with standard ABVD.26 Notably, this analysis was conducted in 2017, when the average sales price of BV was $6970 per 50-mg vial. Although the Consumer Price Index (ie, inflation) for medical care increased by 3.9% between January 2017 and April 2019, the average sales price for BV in the United States increased by 16.3% ($7819 per 50-mg vial as of April 2019) (see Figure 1).22
Average sales price of brentuximab vedotin per dose in the United States. 1Average US sales price for brentuximab vedotin dosed 1.2 mg/kg in a 75-kg patient (ie, two 50-mg single-use vials per dose). CPI, Consumer Price Index.
Average sales price of brentuximab vedotin per dose in the United States. 1Average US sales price for brentuximab vedotin dosed 1.2 mg/kg in a 75-kg patient (ie, two 50-mg single-use vials per dose). CPI, Consumer Price Index.
A second CEA was recently published; its authors reported more favorable results with BV + AVD.27 In that study, Delea et al27 estimated BV + AVD to be associated with greater effectiveness (0.76 vs 0.56 QALYs) and lower incremental costs ($130 706 vs $176 848) than those reported by Huntington et al.26 As a result, Delea et al estimated BV + AVD to have an ICER of $172 074/QALY compared with standard ABVD. This publication27 also provides an estimate based on a subgroup analysis of patients treated in North America that found the hazard ratio (HR) for modified PFS to be 0.60 (95% confidence interval [CI], 0.40 to 0.90; P = .012) compared with 0.83 (95% CI, 0.59 to 1.17; P = .281) in the European region and 0.91 (95% CI, 0.43 to 1.93; P = .810) in the Asian region.28 When using this lower HR in their cost-effectiveness model, Delea et al27 estimated the ICER for BV + AVD compared with ABVD to be $69 442/QALY.
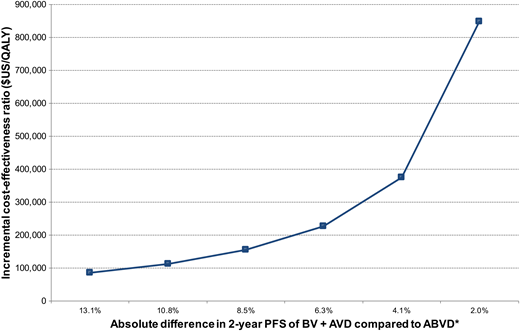
Although the 2 published CEAs of BV + AVD compared with ABVD both used Markov decision analytic modeling over a lifetime horizon, the models differ in their selection of growth factor support, assumptions concerning bleomycin-related mortality, and consideration of long-term declines in survival related to first-line chemotherapy. For example, Delea et al27 assumed that the least expensive growth factor (filgrastim rather than pegylated filgrastim) will be exclusively used and that >50% of patients receiving standard ABVD receive growth factor. The risk of febrile neutropenia with standard ABVD is low, and prophylaxis with growth factors is generally not recommended.29 Thus, their 56% base case estimate is unlikely to be reflective of standard practice and could lead to smaller incremental drug-related expenses associated with BV + AVD than with ABVD.27 Delea et al also estimated lower long-term survival than Huntington et al26 by including excess mortality during remission derived from cHL survival data of individuals treated between 1965 and 1987,30 whereas Huntington et al assumed that patients alive and in remission beyond 5 years have mortality similar to that of age-matched control subjects.26 It is also important to note that the study by Delea et al was sponsored by the manufacturer of BV, with prior work suggesting that published CEAs are more likely to identify favorable ICERs when supported by pharmaceutical organizations.31 Last, the calculated ICERs of both CEA models are highly sensitive to the modified PFS of BV + AVD compared with ABVD, with Huntington et al predicting an ICER of $114 046/QALY if the HR was 0.5 (ie, absolute 2-year PFS difference between BV + AVD and ABVD of 10.8%) (Figure 2).
Incremental cost-effectiveness of BV + AVD compared with ABVD over a range of potential clinical benefit. *Assumes 2-year modified PFS with ABVD of 76.9%. The displayed absolute percentage differences correspond to hazard ratios of 0.4, 0.5, 0.6, 0.7, 0.8, and 0.9.
Incremental cost-effectiveness of BV + AVD compared with ABVD over a range of potential clinical benefit. *Assumes 2-year modified PFS with ABVD of 76.9%. The displayed absolute percentage differences correspond to hazard ratios of 0.4, 0.5, 0.6, 0.7, 0.8, and 0.9.
Cost and cost-effectiveness in the R/R setting
In the post-transplant setting, BV consolidation compared with active surveillance was estimated to produce an ICER of $148 664/QALY after ASCT.32 With the recent 5-year update from the post-ASCT clinical trial showing comparably favorable outcomes in the most at-risk patients (ie, PFS HR, 0.42; 95% CI, 0.30-0.60),33 BV consolidation may be cost-effective in a subgroup of high-risk patients. Outside of BV use in the post-transplant consolidative setting,17 robust clinical data comparing BV with alternatives in the R/R cHL setting are not available. Even less clinical data (ie, durability of response) is available for PD1 blockade to allow robust CEAs in this setting. The lack of prospective multiarm studies for R/R cHL has required regulatory groups to assume key variables during cost-effectiveness modeling. However, recent work incorporating contemporary use of allogeneic stem cell transplant generally found that BV offers ICERs below commonly accepted willingness-to-pay thresholds in the R/R cHL setting.34,35 In fact, the updated appraisal determination for the United Kingdom’s National Institute for Health and Care Excellence estimated an ICER between £16 000 and £18 000 per QALY gained for adults with R/R cHL (equivalent to ∼$21 000/QALY to ∼$25 000/QALY).36 Importantly, the National Institute for Health and Care Excellence appraisal calculated its ICER with BV price set at £2500 (∼$3275) per 50-mg vial, a price that is 58% less than the current average sales price in the United States ($7819).
Special considerations for high-cost drugs and administration in the United States
Although health care systems and payers worldwide must increasingly scrutinize and consider the budgetary impact of novel cancer therapies, the financing and delivery of cancer care in the United States is particularly influenced by rising drug prices. In the United States, office-administered therapies are first purchased by the physician or practice, then billed to patients and/or insurers after drug administration. The margins derived from this “buy and bill” system have historically contributed to >50% of all revenue for US-based medical oncology.37 As both the number and prices of available oncolytics have increased, the upfront expense of maintaining chemotherapy inventory has swelled.38 Furthermore, office-based infusions are reimbursed at higher levels when administered in the hospital setting, including community offices operating (or employed) under the umbrella of a hospital. Unsurprisingly, there has been dramatic consolidation among US oncology practices during the past decade,39 with “vertical integration” between physician-owned offices and hospitals leading the charge.40
Many of these hospitals and integrated outpatient offices also benefit from favorable agreements to purchase drugs at a discount through the 340B Drug Discount Program. This federal program run by the U.S. Department of Health and Human Services through the Health Resources and Services Administration allows participating hospitals to purchase drugs at an average discount of 34%,41 increasing available margins when delivering high-cost novel cancer therapeutics. Although empirical work measuring the influence of financial incentives on cancer therapy use in the United States is limited,42,43 the current reimbursement structure for US providers (and their employers/hospitals) offers strong financial incentives to incorporate high-cost novel therapies into the first-line setting. For a 340B hospital purchasing their medications at an ∼34% discount and then billing insurers for the standard reimbursement, drug-related revenue derived from 6 cycles of ABVD is estimated at $565, compared with nearly $69 022 for 6 cycles of BV + AVD (Table 2).
Novel therapies for first-line cHL are likely here to stay
Although some clinicians specializing in lymphoma are waiting for longer-term follow-up from ECHELON-1 before adopting BV + AVD,44 the use of novel therapies for first-line cHL will likely increase in the coming years. Of the 5 currently accruing or soon-to-open large randomized trials worldwide for first-line cHL, 3 incorporate BV and/or anti-PD1 therapies (Table 3). This includes the US-based S1826 trial (www.clinicaltrials.gov identifier: NCT03907488), which will compare nivolumab + AVD with BV + AVD, electing to forgo ABVD as a comparator arm altogether. This trial enrolls individuals aged 12 years and older, capturing most patients with advanced stage cHL and likely solidifying the use of novel agents in the advanced stage setting in the United States. Smaller, nonrandomized investigations have already explored the use of novel therapies in patients with limited stage cHL, and momentum is likely to build for routinely incorporating novel therapies in the first-line setting, regardless of stage.
Can we afford it?
Because standard chemotherapy (ie, ABVD) is both inexpensive and highly efficacious in the setting of first-line cHL, the addition of high-cost BV and anti-PD1 therapies in this setting is unlikely to represent favorable value under current US pricing. However, because cHL is relatively rare, widespread adoption of these high-cost therapies would lead to relatively modest financial hardship at the system level for wealthy nations. For example, there were ∼7700 new cases of cHL in the United States in 2018, representing less than 0.5% of all new cancers.45 Considering only drug acquisition costs, adopting BV + AVD for all patients with advanced stage cHL in the United States (∼38% of cases, or 2900 individuals) would lead to ∼$584 000 000 higher drug-related expenditures than with ABVD. This is a considerable sum but represents less than 1% of the total $61 billion spent on cancer drugs in the United States in 2017.46
Although the relatively low incidence of cHL reduces the overall budgetary impact of incorporating BV in the first-line setting, this is clearly not the case for modern oncology at large. For example, in the setting of chronic lymphocytic leukemia (CLL), in which oral targeted therapies have recently surpassed immunochemotherapy in terms of efficacy across all major patient subgroups, the costs associated with adopting novel therapies in the first-line setting are substantial. In one model,47 adopting continuous oral targeted therapies across the first-line setting increased the projected annual cost of CLL management in the United States from $0.74 billion to $5.13 billion (590% increase) between 2011 and 2025. The overwhelming majority (96%) of CLL-related costs by 2025 were estimated to be due to drug costs.47 Given the increasing number of cancer settings in which novel immunotherapies and targeted agents are replacing lower-cost cytotoxics, it is not surprising that cancer drug expenditures are expected to increase by 10% to 15% annually.46 Thus, the value proposition and consideration for incorporating BV in the first-line setting for cHL should be viewed as an early exemplar rather than the exception.
Future directions
The average price of new cancer therapies entering the market has risen more than 10-fold during the past 15 years.48 Although it is fair to acknowledge that high drug prices provide strong financial incentives to develop innovative cancer therapies, it is also clear that a continued rise in cancer drug prices is not sustainable. Transparency related to the cost of developing cancer therapies is lacking, but one only needs to look at the 60% increase in cancer drugs entering early clinical trials during the past 10 years to trust that the current return on investment is highly favorable.46 Both the generous reimbursement and the fact that drug prices are not strongly tied to clinical utility have led some to raise concerns that cancer drug development has come at the detriment of investment in other important health care settings.49
A shift toward value-based pricing would better align drug prices with their underlying utility and reward the most innovative cancer therapies. This approach is not new to audiences outside the United States, with most other nations using central health technology assessment bodies to assess the value of new drugs before making national formulary coverage decisions. Using cost-effectiveness modeling, these groups evaluate the available clinical evidence and determine whether a new therapy offers a “reasonable value” compared with alternative therapies. There is no clear consensus of what represents reasonable value in health care; however, there is growing support to use a value between 2 to 3 times the gross domestic product per capita per QALY (ie, ∼$115 000/QALY to ∼$175 600/QALY in the United States).50 Adopting formulary coverage based on transparent health technology assessment fosters meaningful negotiations between payers and manufacturers and is the reason that cancer drug prices are an average of 45% lower outside the United States.51
Indication-specific pricing further recognizes that a given therapy may offer varying clinical utility across clinical settings and allows for value calculations and pricing by clinical indication. In this way, BV in the chemorefractory setting would be reimbursed at a higher price, whereas BV used in the first-line setting would command a significantly lower price. Huntington et al26 estimated that if indication-specific pricing were implemented, acquisition costs for BV used in the first-line setting would need to be reduced by 56% to 73% for ICERs of $150 000/QALY to $100 000/QALY, respectively.
Value-based pricing does have trade-offs, including the need to accept that some drugs with clear clinical efficacy will not be covered. In this circumstance, drugs with reasonable efficacy but unfavorable pricing would not be included in a formulary (nor would they likely be developed). Even for high-value drugs that are eventually deemed to be acceptable for widespread coverage, robust CEAs and price negotiations are time-consuming. This work typically delays access to the novel therapies compared with the current system used in the United States. In the case of BV for cHL, the U.S. Food and Drug Administration (FDA) issued its approval for the first-line setting just 100 days after ECHELON-1 data became publicly available. Insurance regulations in most US states force health insurance companies to cover any FDA-approved cancer therapy, and federal payers (ie, Medicare) have similar provisions. As a result, most insured patients in the United States had access to first-line BV within 4 months of when the 24-month efficacy results were presented. Compare this with the situation in Europe, where approval for marketing came more than 1 year after the release of the efficacy data, and national coverage decisions for first-line cHL are still pending (April 2019). In a recent analysis of European coverage decisions for novel oncologic therapies,52 the median time between European Medicines Agency marketing approval and subsequent coverage decision varied by nation and ranged between 118 (France) and 405 (England) days.
Recognizing these trade-offs and developing an approach that minimizes unintended consequences will be imperative to ensure that value-based drug pricing in the United States improves equity and maximizes access to effective and affordable therapies. Narrower policy alternatives that fail to allow direct payer–manufacturer negotiations or the ability to refuse coverage of a low-value therapy will fall short. For example, the promise of dramatic future cost reductions due to loss of patent protection and exclusivity seems unlikely. The typical 85% to 95% price drop seen when generic small-molecule drugs become available has not been realized in oncology.53 Furthermore, the complexity surrounding many novel therapies has raised concerns about the biosimilar market and its ability to significantly reduce cancer drug spending and bend the cost curve in oncology.54 Fortunately, the setting of cHL continues to have robust and well-designed clinical trials producing actionable comparative data that can inform key stakeholders, including patients, physicians, and payers. Future efforts should ensure transparent reporting of robust comparative data that are able not only to support timely payer coverage decisions but also to allow informed patient-level decisions based on individualized preferences (ie, quality of life, treatment burden, and overall survival).
Treatment selection for the patient
The 36-year-old woman in the clinical case presented for first-line cHL treatment in the spring of 2018, a few months after ECHELON-1 was published. Her IPS score of 2 predicted a relatively favorable outcome with ABVD therapy with estimated 5-year PFS and overall survival at 80+% and 90+%, respectively. The patient had preserved lung function and did not have any specific risk factors for bleomycin toxicity. In discussing the risks and benefits of available treatment options, including BV + AVD, the patient felt most confident with ABVD following a PET-adapted approach, keeping BV as a future option should the need arise. The high cost of BV did surface during conversations, including the potential for out-of-pocket expenses related to her high-deductible health plan. However, her individual cost concerns were not relevant for her decision to proceed with BV + AVD, because she would unfortunately face and meet her maximum annual out-of-pocket expense ($10 000) after routine care, regardless of the cHL treatment she selected.
Conclusions
Multiagent chemotherapy for the treatment of cHL represents a key early success for medical oncology as a specialty. Investigation into cHL continues to provide important and widely applicable insights, including consideration of long-term treatment toxicity, survivorship planning, and application of novel immunotherapies. With relatively well-established treatment paradigms and robust comparative clinical data, first-line cHL is particularly suitable for conducting robust CEAs and exploring value-based pricing. Although incorporating high-cost novel therapies for first-line cHL is unlikely to bankrupt high-income nations, it does serve as an exemplar for addressing the rising costs associated with novel therapies. If stakeholders can eventually agree on how best to balance innovation with affordability and value in first-line cHL, the framework will likely be applicable to the field of modern oncology at large.
Correspondence
Scott F. Huntington, Yale University, 37 College St, New Haven, CT 06510; e-mail: scott.huntington@yale.edu.
References
Competing Interests
Conflict-of-interest disclosure: S.F.H. has received honoraria from Pharmacyclics; consulted for Bayer, Celgene, Genentech, and AbbVie; and received research funding from Celgene, DTRM Biopharma, Debiopharm, and TG Therapuetics.
Author notes
Off-label drug use: None disclosed.