Abstract
The management of superficial vein thrombosis (SVT) is poorly defined and remains controversial overall. SVT has long been considered a benign, self-limited disease, but recent studies show that SVT carries a nonnegligible risk for recurrence, deep vein thrombosis, or pulmonary embolism. Current guidelines recommend the use of low-molecular-weight heparin or fondaparinux, but results of several surveys indicate that the majority of patients with SVT receive nonanticoagulant therapy only, which includes compression stockings or bandages, nonsteroidal anti-inflammatory drugs, topical application of heparin gel, or surgical interventions. However, several recent observational and interventional studies provide better insight into the optimal treatment of patients with SVT who are at different risks for thromboembolic complications. This educational review summarizes the available evidence and aims to provide practical guidance based on a clinical decision pathway.
Learning Objectives
Understand that superficial vein thrombosis (SVT) is a common manifestation of venous thromboembolism that has diagnostic, therapeutic, and prognostic relevance for many patients
Understand that SVT often is associated with concomitant asymptomatic deep vein thrombosis and that every patient with SVT should be considered for an ultrasound examination of the superficial and deep vein system
Understand that patients with SVT without risk factors for thrombus progression do not generally need anticoagulant therapy and that nonsteroidal anti-inflammatory drugs may be sufficient for symptom relief
Understand that patients with SVT with risk factors for thrombus progression need adequate anticoagulant therapy, which may be performed with low doses of oral or parenteral anticoagulants over 30 to 45 days
Understand that progression or recurrence of SVT is not uncommon and can present as deep vein thrombosis or pulmonary embolism; although routine ultrasound follow-up is not necessary for all patients with SVT, those with symptom progression should undergo imaging procedures and should be considered for individualized anticoagulant therapy
Epidemiology of SVT
Superficial vein thrombosis (SVT) is a common condition, with an incidence of 0.3 to 0.6 per 1000 person-years in younger patients and 0.7 to 1.5 per 1000 person-years in older patients.1,2 SVT can occur in every vascular region, including the arm (often as a result of trauma, blood sampling or intravenous injections, or indwelling catheters),3,4 chest, or abdominal veins, but the most common manifestation is in the superficial vein system of the lower extremities.3,4 This review will exclusively focus on lower-limb SVT.
Known risk factors for SVT include varicose veins, obesity, malignancy, age >60 years, history of thrombosis, pregnancy, infection, or smoking.5,6 This risk factor profile is very similar to that of deep vein thrombosis (DVT) or pulmonary embolism (PE), conditions that are commonly summarized as venous thromboembolism (VTE). The Multiple Environmental and Genetic Assessment of Risk Factors for Venous Thrombosis study was a population-based case-control study in The Netherlands, which enrolled 4956 consecutive patients with DVT or PE and 6297 age- and sex-matched controls.7,8 A history of SVT at the time of enrollment was found in 10% of patients with VTE compared with 2% in the control group. Furthermore, patients with a history of SVT had a 6 times higher likelihood of developing DVT and a 4 times higher likelihood of developing PE than controls. As a consequence of this shared risk profile, ∼20% to 25% of patients presenting with SVT have a concomitant DVT or PE at the time of SVT diagnosis,2,5,9 which may be symptomatic or found on screening examinations only.
In the Prospective Observational Superficial Thrombophlebitis (POST) study, 844 patients with confirmed SVT underwent a bilateral complete compression ultrasound to rule out concomitant DVT.10 The prevalence of isolated SVT, concomitant DVT, and PE without DVT was 75.1%, 23.5%, and 1.1%, respectively. Importantly, 17.2% of patients presented with concomitant DVT in the contralateral leg. The authors also demonstrated that DVT was significantly more common in patients in whom SVT affected the trunk of the greater saphenous vein, extended close to the saphenofemoral/popliteal junctions, or affected perforating veins concomitantly.10
However, systematic imaging of both legs in patients with suspected DVT has a very low yield11 and whether routine bilateral ultrasound is justified in every patient with suspected SVT seems questionable.
How I treat: extensive SVT (>5 cm in length) and SVT in nonvaricose veins needs objective confirmation by venous ultrasound, including the examination of the deep veins of the symptomatic leg. In patients with SVT who have a high risk of thromboembolic complications, a bilateral ultrasound may be considered. Furthermore, every patient with SVT should be assessed for symptoms and signs suggestive of PE, because the superficial vein thrombus may have progressed into the deep vein system. Patients with symptoms of PE should undergo objective testing. If patients with SVT are diagnosed with concomitant DVT and/or PE, the type, intensity, and duration of anticoagulant therapy should be guided by the DVT and/or PE.
The following sections of this review will refer only to SVT without concomitant DVT or PE.
Risk factors for thromboembolic complications in SVT
A number of studies have evaluated potential risk factors for thromboembolic complications in patients with SVT. The POST registry with 844 patients with symptomatic lower-limb SVT demonstrated that male sex, history of VTE, previous cancer, and absence of varicose veins were risk factors for thromboembolic complications.5
A very similar risk profile for thromboembolic complications in SVT was identified in the randomized Superficial Thromboembolism Fluxum study.12 During active treatment with low-molecular-weight heparin (LMWH), a previous VTE and/or family history of VTE and absence of varicose veins was associated with VTE and SVT recurrence or extension.
How I treat: At the time of SVT diagnosis, a dedicated assessment of established risk factors for thromboembolic complications is recommended at the time of SVT diagnosis because the type, dosage, and duration of anticoagulant therapy should be tailored to the patient’s characteristics.
Role of thrombophilia in SVT
The prevalence of thrombophilia is high in patients diagnosed with DVT or PE and it seems reasonable to ask whether this association is also present in patients with SVT. In a retrospective database analysis from Italy that included 1294 patients with documented SVT and 1294 matched healthy subjects, Legnani et al.13 demonstrated that the prevalence of coagulation inhibitor deficiencies (1.9% vs 0.15%) and Factor V R506Q Leiden mutation (11.6% vs 4.3%) was significantly higher in patients with SVT. Multiple logistic regression also showed that carrier status of thrombophilia was associated with an increased risk for SVT (odds ratio [OR] for Factor V R506Q Leiden mutation, 3; OR for coagulation inhibitor deficiencies, 12).13 In contrast, G20210A prothrombin mutation, antiphospholipid antibodies, or elevated Factor VIII levels were not found to increase the risk of SVT. Similarly, the Multiple Environmental and Genetic Assessment of Risk Factors for Venous Thrombosis study demonstrated a moderate risk increase for SVT in patients with Factor V Leiden mutation (OR, 2.0), whereas prothrombin G20210A mutation was not associated with a significant risk increase.8
Another study demonstrated a higher prevalence of thrombophilia in patients with primary varicose veins and a history of SVT compared with those without a history of SVT.6 However, several relevant baseline characteristics (including age and body mass index) were statistically different between the groups and the most common findings were PAI-1 and MTHFR polymorphisms, which are not generally accepted to be risk factors for VTE.
Despite this lack of clinical relevance, >15% of respondents in a UK SVT management survey indicated that thrombophilia testing should be part of the diagnostic workup of SVT.14
How I treat: thrombophilia testing is not recommended because the results do not influence SVT management.
Treatment of SVT
The management of SVT is poorly defined and remains controversial overall. SVT has long been considered a benign, self-limited disease3,4,15 but recent studies indicate that SVT carries a nonnegligible risk for SVT recurrence, DVT, or PE.2,5,8,9,16,17 Since 2004, American College of Chest Physicians guidelines have recommended treatment of spontaneous SVT with intermediate dosages of unfractionated heparin (UFH) or LMWH for at least 4 weeks (grade 2B),18 and later guideline updates extended this recommendation to prophylactic doses of fondaparinux over 45 days (grade 2B).19,20
However, ∼10 years later, a global survey among 487 practitioners (predominantly from phlebology or vascular surgery) revealed that only 10% would use anticoagulant treatment in patients with acute SVT in the greater saphenous vein. These treatment rates only increased to 28% in the case of a proximal clot extension to within 10 cm of the saphenofemoral junction and to 20% to 30% in the case of a proximal clot extension during follow-up visits.21 If anticoagulant therapy was considered, treatment durations were reported to be ≤4 weeks in 19% to 23%, up to 3 months in 26% to 31%, and >3 months in 24% to 28%.21
Another study from the United Kingdom used a similar approach and surveyed 369 physicians in charge of SVT treatment decisions.14 In this study, only 20% of respondents would consider LMWH therapy for patients with SVT. From these surveys, it can be concluded that the majority of patients with SVT receive nonanticoagulant therapy only.
Evidence for nonanticoagulant treatment options in SVT
Nonanticoagulant options for SVT therapy include compression stockings or bandages, oral or topical nonsteroidal anti-inflammatory drugs (NSAIDs), topical application of heparin gel, or surgical interventions.22 Results of the above-mentioned UK SVT treatment survey indicated that ∼80% of respondents would use NSAIDs in SVT treatment, 20% would use antibiotics, and only 17% would apply compression therapy.14
In 2014, the results of a single-center randomized controlled trial that compared 3 weeks of compression stocking use vs no compression use in 73 patients with SVT were reported.23 Of note, all included patients also received LMWH at a prophylactic dosage and use of concomitant NSAID therapy was allowed. At 3 weeks, there was no significant difference between the 2 treatment groups with regard to pain reduction, consumption of analgesics, thrombus length, skin erythema, D-dimer, and quality of life; however, at day 7, patients treated with compression stockings had a significantly faster thrombus regression.
A Cochrane analysis summarized the evidence for NSAIDs, topical heparin therapy, and surgery in the treatment of acute SVT.24,25 The authors concluded that compared with placebo, NSAIDs were associated with lower rates of superficial thrombophlebitis extension and/or recurrence (relative risk, 0.46; 95% confidence interval [95% CI], 0.27-0.78) but did not influence the rate of DVT or PE (relative risk, 0.91; 95% CI, 0.25-3.28). Furthermore, surgical treatment combined with compression was associated with a lower rate of DVT and SVT progression compared with compression alone. The majority of studies that compared different oral treatment, topical treatment, or surgery did not report VTE, SVT progression, adverse events, or treatment side effects. The authors concluded that further research is needed to evaluate whether a combination of different interventions is superior to single interventions, which is also indicated by the fact that ∼10% of the study population in the Superficial Phlebitis Treated for 45 Days with Rivaroxaban Versus Fondaparinux (SURPRISE) trial26,27 received short courses of NSAID therapy in addition to anticoagulant therapy despite a lack of evidence for such a combination, which may increase bleeding risk.
How I treat: the evidence for oral or topical NSAIDs, other topical treatments, or surgery is too limited to recommend any of these interventions as a standard treatment of SVT to prevent thromboembolic complications. However, these options may be considered as add-ons to anticoagulant treatment for better symptom control or, in the case of surgery, to treat underlying varicose veins. A single underpowered and confounded study showed that compression stockings did not improve outcomes compared with LMWH and NSAIDs in the treatment of isolated SVT.
Prognosis of SVT without anticoagulant therapy
Both progression or recurrence of SVT and recurrence as DVT or PE are common clinical scenarios in acute SVT.5,8 If, as indicated by SVT management surveys, anticoagulant therapy is not consistently used in patients with SVT, the short- and long-term incidence of thromboembolic complications must be considered.
In 2014, Cannegieter et al.17 reported on the prognostic relevance of SVT using data from a Danish nationwide cohort study. They identified 10 973 patients diagnosed with a first-time SVT in Denmark between 1980 and 2012. During this period, anticoagulant therapy for SVT was not widely used in Denmark; therefore, these data likely allow us to evaluate the natural course of disease in a large cohort of patients with untreated SVT, who were matched in a 1:50 fashion with a total of 515,067 SVT-free patients according to age, sex, and calendar year. During a median follow-up of 7 years, the incidence rate of VTE was 18.0 per 1000 patient-years (95% CI, 17.2-18.9). After adjustment for cancer, pregnancy, fracture, surgery, Charlson Comorbidity Index score, and autoimmune disease, this VTE incidence rate produced a hazard ratio (HR) of 11.3 (95% CI, 10.5-12.1) for DVT and 4.5 for PE (95% CI, 4.1-5.0) compared with the control group, in which the incidence of VTE was 1.2 per 1000 patient-years (95% CI, 1.1-1.2).
As expected, the highest risk for VTE was observed in the first 90 days after SVT diagnosis (incidence rate, 3.4%; 95% CI, 3.0-3.7), with a steadily decreasing HR from 71.4 (95% CI, 60.2-84.7) in the first 3 months after the SVT diagnosis to 5.1 (95% CI, 4.6-5.5) 5 years after the SVT diagnosis. The authors also reported elevated risks for acute myocardial infarction, stroke, and death, but these risk increases were only weak and were not robust in subgroup analyses. Consistent with this, another study by Prandoni et al.28 could not demonstrate an increased risk of arterial events in a 26-month period after SVT, compared with a matched cohort of patients without SVT.
In the Superficial Thrombophlebitis Treated by Enoxaparin (STENOX) trial, 427 patients with acute symptomatic SVT were randomly assigned to receive 40 mg enoxaparin sodium subcutaneously, 1.5 mg/kg enoxaparin subcutaneously, tenoxicam orally, or placebo once daily for 8 to 12 days (Table 1). In the 112 patients with SVT who were treated with placebo only, thromboembolic complications occurred in 35% within 97 days and rates of DVT/PE were as high as 3.6% within the first 12 days.29
The large Comparison of Arixtra in Lower Limb Superficial Vein Thrombosis With Placebo (CALISTO) trial included 3002 patients with SVT who were treated with 2.5 mg fondaparinux once daily or placebo and were followed for up to 77 days (Table 1).16 This trial excluded patients with a very high risk of SVT complications, including individuals presenting with thrombus within 3 cm of the saphenofemoral junction and those with cancer, recent SVT, or DVT/PE. Despite the exclusion of high-risk patients with SVT, the thromboembolic event rates at 45 and 77 days in the placebo arm were 5.9% and 6.3%, respectively.16
Taken together, rates of thromboembolic complications, which mostly consist of SVT progression or recurrence but also include a clinically relevant number of DVT and PE events, are highly variable in observational studies and placebo arms of randomized trials. These differences may relate to different patient risk profiles and also to differences in outcome event definitions and the use of screening for asymptomatic events. This must be considered when event rates for patients with untreated SVT are discussed.
The long-term risk of VTE complications after SVT seems to be comparable to the risk after proximal DVT. This was recently confirmed in the OPTIMEV (Optimization de l’interrogatoire dans l’évaluation du risque thrombo-embolique veineux) study, which prospectively evaluated the long-term risk of VTE recurrence (SVT, DVT, or PE) in patients with SVT without cancer. Compared with patients with proximal DVT, patients with SVT had a similar overall incidence of VTE recurrence (5.4% per patient-year vs 6.5% per patient-year; adjusted HR, 0.9; 95% CI, 0.5-1.6). However, in the case of thromboembolic complications, SVT recurred 6 times more as SVT (2.7% vs 0.6%; adjusted HR, 5.9; 95% CI, 1.3-27.1) and 2.5 times less as deep-VTE events (2.5% vs 5.9%; adjusted HR, 0.4; 95% CI, 0.2-0.9).30
Evidence for anticoagulant treatment in SVT
Anticoagulant treatment options for SVT may include prophylactic or therapeutic dosages of unfractionated heparin, LMWH, vitamin K antagonists, fondaparinux, or direct oral anticoagulants. Over the last 2 decades, several antithrombotic regimens have been studied in the treatment of SVT (Table 1). For instance, different LMWHs have been tested in prophylactic or therapeutic dosages and treatment durations between 10 and 30 days in the Superficial Thrombophlebitis Treated by Enoxaparin trial,29 the VESALIO trial,31 and the Superficial Thromboembolism Fluxum trial.32 In these randomized controlled trials, thromboembolic outcomes including DVT, PE, or recurrent SVT during treatment or follow-up ranged from 2.5% to 22.6%, with higher rates after the end of anticoagulant therapy (Table 1).
In the above-mentioned CALISTO trial, thromboembolic complications occurred significantly less often in patients treated with fondaparinux compared with the placebo group (0.0% vs 5.9% at day 45; 1.2% vs 6.3% at day 77). There was no significant difference between major and minor bleeding between both groups.16
A post hoc analysis of the CALISTO trial evaluated the clinical relevance of SVT extension, which was the main outcome in this study.33 In the placebo arm, symptomatic SVT extension to ≤3 cm from the saphenofemoral junction occurred in 3.6% (of which 9.3% developed subsequent DVT or PE) and extension to >3 cm from the saphenofemoral junction occurred in 3.7% (of which 8.9% developed DVT or PE). In contrast, fondaparinux treatment was associated with significantly lower incidences of SVT extension to ≤3 cm or >3 cm from the saphenofemoral junction (0.3% and 0.8%, respectively) and no subsequent DVT or PE occurred.33
Based on these findings, the 2012 American College of Chest Physicians guideline (updated in 2016) recommends treatment of SVT (with at least 5 cm in length) with prophylactic doses of fondaparinux or LMWH for 45 days (grade 2B) and recommends 2.5 mg fondaparinux daily over a prophylactic dose of LMWH (grade 2C)19,20 ; however, the guideline also indicates that patients who place a high value on avoiding the inconvenience or cost of anticoagulation will be likely to decline anticoagulation.
The prospective randomized SURPRISE trial compared 10 mg rivaroxaban orally vs 2.5 mg fondaparinux subcutaneously over 45 days in selected high-risk patients with above-knee SVT who had additional risk factors for thromboembolic complications, such as male sex, history of DVT/PE, previous or active cancer, systemic inflammatory disease, or SVT in nonvaricose veins.26,27
In the 435 patients included in the per-protocol analysis, thromboembolic complications occurred at day 45 in 3% and 2% of patients in the rivaroxaban and fondaparinux groups, respectively. There were no major bleeds in either group. During active treatment, thromboembolic event rates were comparatively low in both treatment arms for a SVT cohort with a prespecified high-risk profile. At the same time, both treatment groups showed a pronounced increase in thromboembolic complications up to 7% after cessation of anticoagulant therapy. The high event rate in the SURPRISE fondaparinux (comparator) arm (composite end point, 7% at 90 days) indicates that patients with SVT can be stratified by clinical assessment of the risk factor profile at baseline, because this rate was much higher than the event rate for fondaparinux in the lower-risk population in CALISTO (composite end point, 1.2% at 77 days).16,27 The SURPRISE results indicate that patients with SVT who are at high risk for thromboembolic complications may not necessarily need more intense treatment but may need to be treated for >45 days. However, evidence for such an approach is currently lacking.
Although the SURPRISE trial demonstrated the noninferiority of 10 mg rivaroxaban once daily compared with 2.5 mg fondaparinux once daily in the treatment of SVT, it should be noted that outcome event rates during treatment were numerically higher in the rivaroxaban arm, as were rates of clinically relevant nonmajor bleeding events. Therefore, the better convenience of an oral drug and the expected lower costs of 10 mg rivaroxaban once daily compared with 2.5 mg fondaparinux once daily in many health care systems must be balanced against a slightly higher event rate.
Additional considerations in SVT treatment decisions
Side effect profile of anticoagulants
Apart from bleeding complications, other side effects of systemic drugs (e.g., allergic reactions) or class-specific complications (e.g., heparin-induced thrombocytopenia type II [HIT]) must be considered in decision making.
Although the absolute risk for HIT seems to be very low during SVT treatment,34 patients should be informed about this risk and the need for regular blood count checks. However, nonheparin anticoagulants (e.g., fondaparinux or rivaroxaban) do not carry a risk for HIT,16,27 which also makes them an alternative for patients with SVT with a history of HIT.
Treatment burden
The most widely used anticoagulant therapies for SVT (namely, LMWH and fondaparinux) have limitations as a result of the need for self-injections, which may result in poor patient compliance. Oral anticoagulants may overcome the burden of self-injections but they similarly carry the risk of adverse drug reactions or bleeding complications, which contribute to the burden of anticoagulant therapy for the patient.
Costs and cost-effectiveness of anticoagulants in SVT
Even for a short course of oral or parenteral anticoagulation, treatment costs are not negligible. A post hoc analysis of the CALISTO trial demonstrated that a 45-day course of prophylactic fondaparinux in the prevention of thromboembolic complications in patients with SVT is not cost-effective.35 The authors suggested that “a better value for money may be obtained in patients with a higher risk of thrombotic complications.”35 In some jurisdictions, the costs of both oral and parenteral anticoagulants will be a disincentive to their use.
Follow-up of patients with SVT
As stated above, SVT shares many risk factors with DVT and PE, including cancer. Therefore, it seems reasonable to ask whether the prognostic relevance of idiopathic DVT/PE to predict occult cancer is similar for SVT. In a large observational study using linked Danish nationwide health databases, standardized incidence rates (SIRs) of cancer after a diagnosis of SVT, DVT, or PE were assessed.36 In the first year after VTE diagnosis, SIRs were 2.5 2.8, and 3.3 in patients with SVT, DVT, and PE, respectively. SIRs decreased to 1.1 for SVT, DVT, and PE after 1 year. It could be concluded from these data that a search for hidden cancer may be considered in some patients with SVT if no other explanations (e.g., varicose veins) are present. On the other hand, an extensive search for malignancy has been shown to provide no benefit in patients with unprovoked DVT or PE37-39 or after a first SVT event.40 Therefore, cancer screening in patients with unprovoked SVT should only be applied after a careful clinical assessment of cancer probability such as patients with unexplained recurrent SVT in nonvaricose veins.
Patients with SVT may develop DVT or clot extension or recurrence, which raises the question of whether routine ultrasound follow-up is needed. In the above-mentioned POST study, 537 patients with confirmed SVT underwent a compression ultrasound of the superficial and deep vein system between 8 and 15 days after the initial ultrasound.41 Patients without a clinical suspicion of a thromboembolic complication were found to be free of events in 97.7% of cases. The authors concluded that a routine follow-up compression ultrasound detected few asymptomatic venous thrombotic complications but failed to identify patients at risk of thromboembolic events during follow-up and therefore was neither efficient nor cost-effective.41
How I treat: suggestions for an SVT treatment algorithm
The following conclusions can be summarized from the above-mentioned evidence:
Patients with SVT may have concomitant DVT or PE at the time of SVT diagnosis. Consequently, patients with extensive SVT (>5 cm in length) and SVT in nonvaricose veins need objective confirmation by a venous ultrasound, including the examination of the deep veins of the symptomatic leg. In patients with SVT who are at high risk of thromboembolic complications, a bilateral ultrasound may be considered. Furthermore, patients should be assessed for signs and symptoms of PE, which would need to be confirmed with objective testing.
Patients with concomitant DVT or PE should be treated as DVT or PE and the manifestation of SVT would only need symptomatic therapy.
Patients with SVT in close proximity to the deep vein system should be treated as DVT and therapeutic anticoagulation is warranted.
If DVT and PE can be safely ruled out, the decision for or against anticoagulant treatment should be based on the exact localization and extent of the SVT: small clots or clots in side branches of the saphenous veins do not require anticoagulant treatment.
Patients with more extensive SVT may also not need anticoagulant therapy if no additional thromboembolic risk factors (e.g., male sex, history of DVT/PE, cancer, systemic inflammatory disease, or SVT in nonvaricose veins) are present.
Patients with SVT of at least 5 cm and additional thromboembolic risk factors (see above) should be treated with prophylactic dosages of anticoagulants for a period of at least 45 days. Fondaparinux (2.5 mg subcutaneously once daily) or rivaroxaban (10 mg orally once daily) have demonstrated high efficacy and safety in this setting.
Routine ultrasound follow-up is not necessary for patients with SVT, but clinical suspicion of SVT progression or recurrence should be objectively confirmed by an ultrasound, which again should include the deep vein system.
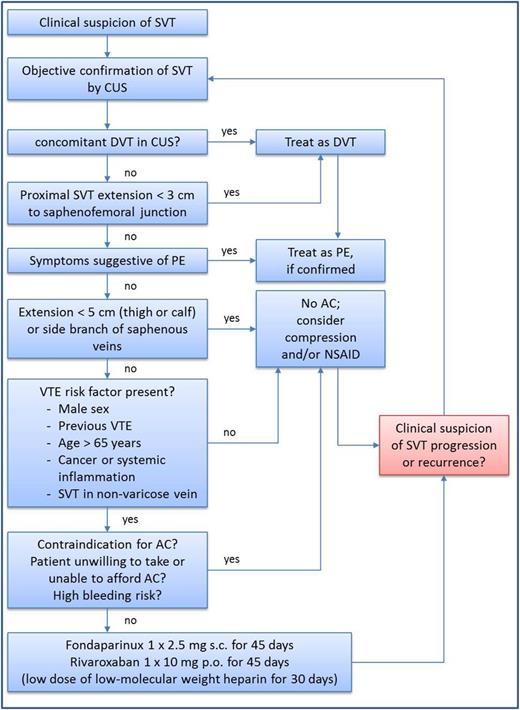
A clinical pathway is summarized in Figure 1. However, it should be noted that this treatment algorithm has not been formally validated.
Treatment decision algorithm for patients with SVT. AC, anticoagulation; CUS, compression ultrasound; DVT, deep vein thrombosis; NSAID, nonsteroidal anti-inflammatory drug; PE, pulmonary embolism; SVT, superficial vein thrombosis; VTE, venous thromboembolism.
Treatment decision algorithm for patients with SVT. AC, anticoagulation; CUS, compression ultrasound; DVT, deep vein thrombosis; NSAID, nonsteroidal anti-inflammatory drug; PE, pulmonary embolism; SVT, superficial vein thrombosis; VTE, venous thromboembolism.
Correspondence
Jan Beyer-Westendorf, Thrombosis Research Unit, Division of Hematology, Department of Medicine I, Carl Gustav Carus University Hospital, Technische Universität Dresden, Fetscherstrasse 74, 01307 Dresden, Germany; e-mail: jan.beyer@uniklinikum-dresden.de.
References
Competing Interests
Conflict-of-interest disclosure: J.B.-W. has received research funding and honoraria from Bayer, Boehringer Ingelheim, Daiichi-Sankyo, and Pfizer.
Author notes
Off-label drug use: None disclosed.