Abstract
In recent years, several new drugs have been approved for the treatment of multiple myeloma. Many of these newer drugs are highly efficacious and less toxic than older chemotherapy drugs. In 2014, the diagnostic criteria for multiple myeloma were revised. The intent with the new criteria was to identify patients who require therapy at an earlier stage than at manifestation of organ complications. A subset of patients who were previously defined as having high-risk smoldering multiple myeloma was redefined as having multiple myeloma. In this context, it is logical to raise questions regarding the optimal clinical management of patients who are diagnosed with smoldering multiple myeloma in the current era. When is the optimal time to start therapy? Do the clinical trajectories for patients suggest there are distinct sub-entities hidden in the current category of smoldering multiple myeloma? How can we move the field forward from here? This paper reviews and dissects data and models on the topics of clinical features, underlying biology, and early treatment trials in smoldering multiple myeloma. The text highlights assumptions, facts, and gaps in the literature. As indicated in the title of the paper, the recurrent theme of the text is this: shall we treat smoldering multiple myeloma in the near future?
Learning Objectives
To understand the current definition of smoldering multiple myeloma and how it was introduced in the literature
To dissect distinct clinical trajectories for patients in the current category of smoldering multiple myeloma
To review data from intervention studies targeting patients with smoldering multiple myeloma and to address weaknesses and gaps in the literature
To discuss goals and limitations of intervention studies for patients with smoldering multiple myeloma and how to design future studies in this setting
Introduction
Despite emerging data that suggest early initiation of treatment may be the future direction for improved clinical outcomes, the current standard of care is still “watch and wait” for patients with smoldering multiple myeloma.1 In recent years, several new drugs have been approved for the treatment of multiple myeloma, and many of these drugs are highly efficacious and less toxic than older chemotherapy drugs.2 These facts raise many questions regarding the optimal clinical management of patients diagnosed with smoldering multiple myeloma. In the absence of well-defined guidelines and due to lack of solid data about this topic, I have added my expert opinions and recommendations going forward (these are consistently highlighted for full transparency). The overall scope of the paper is to critically review and dissect data and models on the topics of clinical features, underlying biology, and early treatment trials in smoldering multiple myeloma. The text highlights assumptions, facts, and gaps in the literature. As indicated in the title of the paper, the recurrent theme of the text is this: shall we treat smoldering multiple myeloma in the near future?
The structure of the paper is as follows. First, it gives an overview of how the term “smoldering multiple myeloma” initially was introduced in the literature and how we have arrived at our current positions on this topic. Second, it discusses data from early intervention studies targeting patients with smoldering multiple myeloma, and it addresses weaknesses and gaps in the literature. Finally, it raises questions on the goals of early intervention and how to advance the field in this setting.
What is smoldering multiple myeloma?
From smoldering acute leukemia to smoldering multiple myeloma: clinical observations
In 1963, based on clinical observations, Rheingold and colleagues introduced the terminology “smoldering acute leukemia.”3 In their case series, they described 3 patients who present with an atypical variant of acute leukemia, a clinical picture of low-grade intensity as it smolders along, symptoms of fatigue or spontaneous bruising for months to years, and unremarkable physical examination except for pallor and ecchymoses. The authors stated that “…smoldering acute leukemia is a variant of acute leukemia characterized by a prolonged and often more benign course. The onset is insidious, often with a history going back several years, usually with an unexplained anemia although thrombocytopenia and leukopenia have been present alone or in combination. During the smoldering phase the clinical picture is mild.”3
Inspired by the ideas of Rheingold and colleagues,3 Kyle and Greipp reviewed all patients diagnosed with multiple myeloma at Mayo Clinic before 1974 (N = 334), permitting at least 5 years of follow-up when writing their paper in 1980. They identified 6 cases that fulfilled criteria for the bone marrow and blood-based diagnosis of multiple myeloma, but they had not developed anemia, lytic bone lesions, hypercalcemia, renal failure, or other manifestations of multiple myeloma during an observation period of 5 or more years (Table 1). In 1980, in their research letter with a case series of 6 patients, Kyle and Greipp described these patients as having “smoldering multiple myeloma… analogous to smoldering acute leukemia” because the patients had 10% or more plasma cells in the bone marrow and a monoclonal-(M)-protein of at least 3 g per deciliter in the serum but yet remained stable without specific therapy for 5 or more years.4 At the end of their research letter, Kyle and Greipp stated that “… although chemotherapy may prevent or lessen the complications of active myeloma, therapy may lead to leukopenia, thrombocytopenia, refractory anemia, and acute leukemia. Furthermore, unnecessary chemotherapy causes unnecessary expense, and it is a source of concern to the patient. This form of multiple myeloma, although uncommon, should be recognized, and the patients should be observed without chemotherapy.”4
As mentioned above, the clinical observations of what resulted in the proposed clinical entities smoldering acute leukemia and smoldering multiple myeloma were based on patients with characteristics of acute leukemia and multiple myeloma, respectively, but without development of clinical symptoms consistent with these diseases during several years of follow-up. In the proposed “smoldering multiple myeloma” case series, the patients had M-protein concentrations of 3.0 to 3.6 g/dL and bone marrow plasma cell (BMPC) infiltrations of 10% to 17%, and they were followed between 5 and 16 years without clinical progression (Table 1).4
First diagnostic consensus criteria of smoldering multiple myeloma in 2003
In 2003, 23 years after the initial Mayo Clinic case series mentioned above,4 the International Myeloma Working Group (IMWG) developed a consensus definition for smoldering multiple myeloma.5 The delay was mostly because few available drugs (alkylating agents and steroids) for the treatment of multiple myeloma were available; therefore, there was no strong clinical interest in this topic. These drugs were quite toxic and with limited efficacy. The clinical perception was that it was better to wait until the patient was in direct need for therapy. During this era, multiple myeloma was defined by certain symptoms, reflective of multiple myeloma causing complications in organ systems, including the skeleton, the kidneys, and, systemically, hypercalcemia (serum calcium ≥11.5 mg/dL), renal failure (defined by creatinine ≥1.95 with no other etiology), anemia (hemoglobin ≤10 g/dL or >2 g/dL below the lower limit of normal), or lytic bone lesions (lytic lesions by X-ray of the skeleton, osteoporosis with pathologic fractures, or cord compression). In the 2003 IMWG criteria, smoldering multiple myeloma was defined as serum M-protein ≥3 g/dL and/or ≥10% monoclonal plasma cells in the bone marrow in the absence of the above criteria.5
Retrospective cohort studies from Mayo Clinic: setting the stage
To build on the initial case series with 6 patients who were identified from all patients (N = 334) diagnosed with multiple myeloma at Mayo Clinic before 1974,4 the Mayo Clinic group continued to document its own clinical data on patients with similar characteristics. In 2007, Kyle and colleagues conducted a retrospective cohort study of 276 patients with smoldering multiple myeloma seen at Mayo Clinic between 1970 and 19956 ; the study suggests an average risk of progression to multiple myeloma of about 10% per year and the observation of high-, intermediate-, and low-risk groups for progression.6 In an updated version of their study, the risk groups of progression were based on additional serum markers.7 Although these data are inherently limited by the retrospective study design, they have played an important role to set the stage for the field. They have stimulated many other researchers to develop prospective studies, translational research studies, and early intervention studies, which are discussed in more detail below.
Influence of new multiple myeloma guidelines in 2014
In 2014, the IMWG updated its criteria for multiple myeloma.8 The intent with these new criteria was to identify multiple myeloma requiring therapy at an earlier stage than at manifestation of organ complications. A handful of smaller, retrospective studies focusing on patients with smoldering multiple myeloma found certain biomarkers to be predictive of transformation to multiple myeloma within a short period of time. These biomarkers led to the revised updated criteria for multiple myeloma. The new biomarkers are (1) elevated serum free light chains (sFLC) with a sFLC ratio of 100 or higher (the involved light chain has to be 10 mg/dL or higher), (2) 60% or more light chain–restricted plasma cells in the bone marrow, and (3) 2 or more focal bone marrow lesions in the skeleton as determined by magnetic resonance imaging (MRI) (Table 2).9
To better understand the context, it is necessary to review underlying original data. The above arbitrary cutoffs were initially reported in retrospective single-center studies of patients with smoldering multiple myeloma. It was observed that the biomarkers mentioned above were associated with, on average, around 1 year progression time from smoldering multiple myeloma to multiple myeloma. For example, in an expanded follow-up study of the Mayo Clinic cohort, the authors retrospectively studied the predictive value of the sFLC assay in 586 patients with smoldering multiple myeloma diagnosed between 1970 and 2010.10 A serum involved/uninvolved free light chain (FLC) ratio ≥100 was observed in 15% (n = 90) of the total cohort, and the risk of progression the first 2 years among these patients was 72%. The authors concluded these patients to be candidates for early treatment intervention.10 In another follow-up paper on the Mayo Clinic retrospective cohort, the authors observed that 6 of the 276 (2%) patients with smoldering myeloma progressed to multiple myeloma within 3 to 9 months; these 6 patients had a plasma cell infiltration of the bone marrow to be 60% or higher.11 They also expanded the search in their database to include all patients with smoldering multiple myeloma diagnosed at their institution between 1996 and 2010 (N = 655); in 21 of these they found the plasma cell infiltration of the bone marrow to be 60% or higher.11 The original study on the role of MRI was done by Hillengass and colleagues, who used whole-body MRI in a retrospective series of 149 patients with smoldering myeloma.12 They showed that among those with 2 or more focal lesions (N = 23 of 149, 15%) in the bone or bone marrow, 12 of 23 (50%) progressed to multiple myeloma within 13 months and 16 of 23 (70%) progressed to multiple myeloma within 2 years.12 Subsequently, smaller efforts were launched to replicate these observations. For example, a Greek study group assessed 67 patients with smoldering myeloma with MRI of the spine and the pelvis; 9 of 67 (14%) patients had 2 or more focal lesions, and all 9 progressed to multiple myeloma within 4 years13 (see the 2014 IMWG updated criteria for all details and references). Taken together, upon review of these reports the IMWG consensus group felt it was clinically justifiable to integrate these biomarkers, to be written up by a writing committee, and launched as the updated IMWG diagnostic criteria for multiple myeloma requiring therapy.8 On a clinical note, the bottom line is that the 2014 IMWG definition of multiple myeloma requiring therapy states that patients with 1 or more of the defined 7 variables (ie, the former 4 clinical criteria and the new 3 biomarkers) fulfill the definition of multiple myeloma requiring therapy (Table 2).8
With the introduction of the 2014 IMWG criteria for multiple myeloma,8 a proportion of patients previously considered to have smoldering multiple myeloma now fulfill the criteria of multiple myeloma requiring therapy. Ironically, with the change of the multiple myeloma criteria, the patients sometimes referred to as “ultra high risk smoldering multiple myeloma” (ie, very high risk of developing multiple myeloma) have become “ultra low risk multiple myeloma” because they now represent the earliest stage of multiple myeloma (ie, with a better likelihood of good clinical outcomes).14 Again, with the change of the definition of multiple myeloma in 2014, multiple myeloma no longer is a disease that requires symptoms. The old terminology “asymptomatic multiple myeloma” has become irrelevant (because it includes both smoldering multiple myeloma and some patients with multiple myeloma) and the currently correct terminologies are smoldering multiple myeloma and multiple myeloma requiring therapy.
How common is smoldering multiple myeloma in the general population?
The incidence and prevalence of smoldering multiple myeloma in the population is not well defined. The best available estimates to date come from a recent study by Kristinsson and colleagues who used the Swedish Myeloma Registry from 2008 to 2011 with a total of 2494 patients with any type of newly diagnosed myeloma showing that 360 (14.4%) had smoldering multiple myeloma.15 Among patients with smoldering multiple myeloma, 104 (28.8%) had high-risk smoldering multiple myeloma as defined by Mayo Clinic criteria (with an average time of about 2 years until progression to multiple myeloma; see further details of risk categories below).15 Thus, in the Swedish Myeloma Registry the patients with high-risk smoldering multiple myeloma accounted for 4.2% of all patients with any type of newly diagnosed myeloma. With use of the world population as reference, the age-standardized incidence of smoldering multiple myeloma was determined to be 0.44 cases per 100 000 person-years and the incidence of high-risk smoldering multiple myeloma to be 0.14 cases per 100 000 person-years.15 As a point of reference, in the United States the age-standardized incidence of multiple myeloma is 6.6 per 100 000 person-years.16 Most important, given the facts that the smoldering multiple myeloma is an asymptomatic condition17 and that a large prospective cancer screening trial (N > 77 000) has showed that all cases of multiple myeloma are preceded by a precursor stage including smoldering multiple myeloma,18 the numbers and incidence rates on smoldering multiple myeloma from the Swedish Myeloma Registry are presumably largely underestimated because the population has not been screened and the patients with smoldering myeloma are asymptomatic. An ongoing population-based, nationwide myeloma precursor screening study targeting all Icelanders 40 years or older (N ∼ 140 000) will provide answers based on prospective data in the coming few years.
Clinical interpretations of available retrospective data
Based on the first retrospective original cohort study from the Mayo Clinic published in 2007, the overall risk of progression from smoldering multiple myeloma to multiple myeloma was observed to be about 10% per year for the first 5 years, 3% per year for the next 5 years, and 1% per year for the last 10 years, suggesting that their definition of smoldering multiple myeloma is clinically heterogeneous.6 Based on these patterns, the 2010 IMWG guidelines for clinical management of smoldering multiple myeloma recommend patients to be monitored with blood tests every 2 to 3 months for the first year, followed by every 4 to 6 months for 1 year, with eventual 6- to 12-month evaluations if clinically stable thereafter.17 The 2010 IMWG guidelines recommend closer follow-up of patients with higher risk clinical characteristics.17
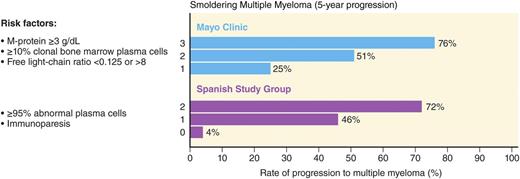
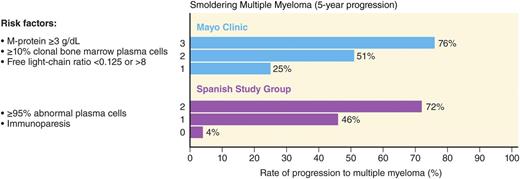
The current clinical factors associated with risk of progression are mainly based on the “level of tumor burden” in these patients assessed by the degree of tumor involvement in the bone marrow and the quantification of monoclonal protein in the peripheral blood (ie, higher tumor infiltration of the bone marrow and higher concentration of monoclonal protein in the blood confer higher risk, reflective of higher tumor burden). The 2 most widely used clinical risk stratification methods are the Mayo Clinic7 and the PETHEMA19 (the Programa para el Tratamiento de Hemopatias Malignas) classifications (Figure 1).19 The Mayo Clinic criteria are based on the levels of serum protein markers (serum protein electrophoresis and FLC assay) and the percentage of BMPCs in the bone marrow.7 The risk stratification of PETHEMA focused on the use of multi-parameter flow cytometry of the bone marrow to quantify the ratio of abnormal, neoplastic plasma cells (aPC) to normal plasma cells in the bone marrow, and reduction of uninvolved immunoglobulins in peripheral blood.19 Both of these models are based on small numbers (Mayo Clinic, N = 273, and PETHEMA, N = 93) derived from retrospective cohorts.7,19 A recent head-to-head comparison of the Mayo Clinic and PETHEMA models in a prospective cohort of 77 patients with smoldering multiple myeloma identified 38, 35, and 4 patients as low-, intermediate-, and high risk by the Mayo Clinic model.20 In the same cohort, the PETHEMA model classified 17, 22, and 38 patients as low-, intermediate-, and high risk, respectively. There was significant discordance in overall patient risk classification (only 28.6% concordance) and in classifying patients as low vs high (P < .0001), low vs non-low (P = .0007), and high vs non-high (P < .0001) risk.20 This illustrates the need for prospectively validated models to characterize individual patient risk of transformation to multiple myeloma.
Risk stratification schemes for smoldering multiple myeloma. Two major models for risk stratification are the Mayo Clinic and the PETHEMA models. The Mayo Clinic model focuses largely on serum protein abnormalities. For smoldering myeloma patients, the following features are considered to be adverse risk factors: >3 g/dL M protein, an FLC ratio outside the reference range of 0.125 to 8, and >10% BMPCs. The PETHEMA model uses multiparametric flow cytometry of bone marrow aspirates to differentiate aberrant from normal plasma cells (aPC versus BMPC). Plasma cells characteristically express CD138 and intense (bright) CD38. The features of aPCs included decreased CD38 expression, expression of CD56, and the absence of CD19 and/or CD45. In their study, smoldering multiple myeloma patients with >95% phenotypically aPC of total BMPC (ie, >95% aPC/BMPC) at diagnosis had a significantly higher risk of multiple myeloma progression. In addition, they characterized uninvolved immunoglobulins in peripheral blood in relation to risk of progression to multiple myeloma. For smoldering multiple myeloma patients, the risk factors in their model are >95% aPCs/BMPC and immunoparesis. Adapted from Landgren et al.49
Risk stratification schemes for smoldering multiple myeloma. Two major models for risk stratification are the Mayo Clinic and the PETHEMA models. The Mayo Clinic model focuses largely on serum protein abnormalities. For smoldering myeloma patients, the following features are considered to be adverse risk factors: >3 g/dL M protein, an FLC ratio outside the reference range of 0.125 to 8, and >10% BMPCs. The PETHEMA model uses multiparametric flow cytometry of bone marrow aspirates to differentiate aberrant from normal plasma cells (aPC versus BMPC). Plasma cells characteristically express CD138 and intense (bright) CD38. The features of aPCs included decreased CD38 expression, expression of CD56, and the absence of CD19 and/or CD45. In their study, smoldering multiple myeloma patients with >95% phenotypically aPC of total BMPC (ie, >95% aPC/BMPC) at diagnosis had a significantly higher risk of multiple myeloma progression. In addition, they characterized uninvolved immunoglobulins in peripheral blood in relation to risk of progression to multiple myeloma. For smoldering multiple myeloma patients, the risk factors in their model are >95% aPCs/BMPC and immunoparesis. Adapted from Landgren et al.49
Other risk factors that have been examined include the role of IgA (vs IgG) isotype, the presence of proteinuria, circulating plasma cells, a high proliferative rate of BMPCs, and abnormal MRI findings.21-23 Also, studies have reported that chromosomal abnormalities present in the plasma cells are also critical for the rate of progression in smoldering multiple myeloma. Two studies showed that the presence of deletion 17p or t(4,14) is associated with the shortest time to progression and that trisomies were a risk factor for progression from smoldering multiple myeloma to multiple myeloma.24,25 Gains of 1q21 were also associated with increased risk for progression among patients with smoldering multiple myeloma. Similar to the Mayo Clinic and the PETHEMA models, these models are also derived from the limited series of retrospective patients. Currently, it is unknown which model is better. Overall, as mentioned above, there is a need for prospectively validated markers and models to define the individual patient’s risk of progressing to multiple myeloma. The above mentioned Icelandic screening study will provide the first insights on this topic based on population-based, prospective data. When available, the results from the Icelandic study are anticipated to replace existing retrospective models in the literature.
Dissecting Mayo Clinic retrospective data
As indicated above, the key message from the above-mentioned 2 original retrospective studies from the Mayo Clinic is that the current definition of smoldering multiple myeloma is a highly heterogeneous clinical entity. Looking at the patterns of progression from smoldering multiple myeloma to multiple myeloma in the 2007 Mayo Clinic study,6 in my opinion, I think 3 main clinical patterns exist (Figure 2).
Dissecting Mayo Clinic retrospective data from smoldering multiple myeloma to multiple myeloma: 3 patterns of clinical trajectory. When the patterns of progression from smoldering multiple myeloma to multiple myeloma in the retrospective Mayo Clinic study are looked at (A),6 as discussed in detail in the text, in my opinion, this definition is highly clinically heterogeneous, and it could be dissected into 3 main clinical patterns: multiple myeloma with early detection (B), indolent multiple myeloma (C), and monoclonal gammopathy (D). That patients with early detection multiple myeloma, typically, develop multiple myeloma within 1 to 2 years indicates that they have the same disease as patients with multiple myeloma, just at an earlier stage.7,19 As discussed in the text, to me, it seems logical to propose that the clinical implication of these arguments is that patients with high-risk smoldering multiple myeloma should be moved into the definition of multiple myeloma so they can be offered access to active therapy. Regarding the role of early treatment in the group of patients with indolent multiple myeloma, it is less obvious to form strategies. Reviewing the literature on indolent lymphomas,33 for example, early intervention vs delayed intervention (eg, at rising biomarkers) are both options that may be proposed. We need properly designed clinical trials to obtain answers on optimal management of indolent multiple myeloma. Also as discussed in detail in the text, with new data that link the precursor and multiple myeloma together,18 the “uncertain significance” (ie, MGUS) terminology seems no longer relevant. To me, it seems reasonable to propose the terminology “monoclonal gammopathy” for current patients with MGUS and patients with low-risk smoldering multiple myeloma. To caution the reader and to avoid confusion in the clinical field, before going forward, these ideas and concepts will have to be agreed on and endorsed in a consensus document. The intent is to share expert opinions based on clinical experience and original data, beyond the regular text book. The hope is to stimulate the reader to approach the current paradigm in a critical manner and to facilitate avenues for new translational research. Panel A is adapted from Kyle et al.6
Dissecting Mayo Clinic retrospective data from smoldering multiple myeloma to multiple myeloma: 3 patterns of clinical trajectory. When the patterns of progression from smoldering multiple myeloma to multiple myeloma in the retrospective Mayo Clinic study are looked at (A),6 as discussed in detail in the text, in my opinion, this definition is highly clinically heterogeneous, and it could be dissected into 3 main clinical patterns: multiple myeloma with early detection (B), indolent multiple myeloma (C), and monoclonal gammopathy (D). That patients with early detection multiple myeloma, typically, develop multiple myeloma within 1 to 2 years indicates that they have the same disease as patients with multiple myeloma, just at an earlier stage.7,19 As discussed in the text, to me, it seems logical to propose that the clinical implication of these arguments is that patients with high-risk smoldering multiple myeloma should be moved into the definition of multiple myeloma so they can be offered access to active therapy. Regarding the role of early treatment in the group of patients with indolent multiple myeloma, it is less obvious to form strategies. Reviewing the literature on indolent lymphomas,33 for example, early intervention vs delayed intervention (eg, at rising biomarkers) are both options that may be proposed. We need properly designed clinical trials to obtain answers on optimal management of indolent multiple myeloma. Also as discussed in detail in the text, with new data that link the precursor and multiple myeloma together,18 the “uncertain significance” (ie, MGUS) terminology seems no longer relevant. To me, it seems reasonable to propose the terminology “monoclonal gammopathy” for current patients with MGUS and patients with low-risk smoldering multiple myeloma. To caution the reader and to avoid confusion in the clinical field, before going forward, these ideas and concepts will have to be agreed on and endorsed in a consensus document. The intent is to share expert opinions based on clinical experience and original data, beyond the regular text book. The hope is to stimulate the reader to approach the current paradigm in a critical manner and to facilitate avenues for new translational research. Panel A is adapted from Kyle et al.6
Multiple myeloma, early detection.
The group with the shortest time of follow-up from smoldering multiple myeloma diagnosis to multiple myeloma diagnosis is truly reflective of “multiple myeloma, early detection”. That these patients develop multiple myeloma within 1 to 2 years indicates they have the same disease as patients with multiple myeloma, just at an earlier stage.7,19 This is very similar to many other conditions clinicians manage in their daily practices.
When the patterns of progression from smoldering multiple myeloma to multiple myeloma in the 2007 retrospective Mayo Clinic study6 are looked at and implications of improved diagnostics, which we have access to in our clinics today, are considered, the following outcomes seem very likely: some patients in this category already have (undetected) disease that could be defined by modern testing, whereas others are negative by modern testing when conducted the first time but will be detectable at subsequent testing(s).
Several important clinical aspects should be mentioned in this context. In the 2003 IMWG criteria, multiple myeloma bone disease was defined as “lytic lesions by X-ray of the skeleton, osteoporosis with pathologic fractures, or cord compression.”5 The updated 2014 IMWG criteria for multiple myeloma recommend the use of either skeletal X-ray, or positron emission tomography/computed tomography (PET/CT), or low-dose CT.8 The reason why a range of options is given in the 2014 criteria is to allow for international variations in terms of access to imaging. From the standpoint of sensitivity, it should be emphasized that skeletal X-ray requires the loss of 30% to 50% of the bone mass before it detects lesions.26 As a point of reference, among patients with smoldering multiple myeloma with high-risk clinical features defined by either the Mayo Clinic7 or the PETHEMA19 classifications mentioned above, despite having negative skeletal X-rays, about 30% of these patients have detectable bone lesions by PET/CT.27 For clinicians who see a patient with suspected smoldering multiple myeloma in a clinic, it is important to be aware of the details of the 2014 IMWG definition of multiple myeloma. Clinicians need to ensure the patient is fully worked up with the new biomarkers (discussed in detail above; see also Table 2) to rule out multiple myeloma requiring therapy.8
As stated above, for patients with high-risk smoldering multiple myeloma, the average progression time to multiple myeloma is short. After careful assessment, which has been described previously, for patients without detectable disease, the risk of developing multiple myeloma still remains high (based on current knowledge, overall there is ∼75% risk within 5 years for patients with high-risk smoldering multiple myeloma). If there were drugs approved by the US Food and Drug Administration (and European Medicines Agency and equivalent agencies in other parts of the world) to treat these patients, from a clinical perspective it seems logical to propose that early treatment (same as multiple myeloma) should be offered to patients with early detection multiple myeloma before they develop complications of the disease (such as acute kidney injury, cord compression, dialysis use, fracture, and hypercalcemia),28 and, presumably, with a high likelihood of having good clinical outcomes.14,29,30 The past years, several new drugs have been approved for the treatment of multiple myeloma, and several of these drugs are highly efficacious and less toxic than older chemotherapy drugs.2
Going forward, to me, it seems logical to propose that the clinical implication of these arguments is that patients with high-risk smoldering multiple myeloma should be moved into the definition of multiple myeloma so they can be offered access to active therapy (Table 3). As always, one may argue that we need more solid data (ie, prospective studies) before changes to the current terminology be considered. On the other hand, it is correct to conclude that our current clinical terminology is built on the same level of evidence as these proposed ideas.8-12 Indeed, given that the updated 2014 IMWG multiple myeloma criteria included 3 biomarkers, which were extrapolated from small retrospective cohorts, it seems fair to conclude that “we have already crossed that bridge.” The future will tell us when the field is ready to move forward. To avoid confusion in the clinical field, before going forward, this will have to be agreed on and endorsed in a consensus document.
Indolent multiple myeloma.
The group with an extended time-window (about 5-10 years) from smoldering multiple myeloma diagnosis to multiple myeloma diagnosis6 appears to be reflective of a group with indolent progression, or “indolent multiple myeloma” (Table 3). To me, it seems logical to propose that they are reflective of a more indolent disease biology, which takes longer to evolve into the current multiple myeloma criteria.
As an analogy, based on many years of clinical experience with patients with multiple myeloma, a proportion of all patients with multiple myeloma I treat and monitor in my clinic have more indolent disease biology. Compared with other patients with multiple myeloma with more active biology, many patients with indolent multiple myeloma biology often times have slower onset of symptoms, which typically are less profound at disease presentation; they respond slower to therapy, and they also relapse slower (this picture is not true for all patients, and the dynamics of a patient with indolent disease at diagnosis may change and become more active at relapse, which in turn is likely driven by acquired mutations and/or clonal selection due to treatment pressure). It seems reasonable to conjecture that the massive genetic heterogeneity observed in patients with multiple myeloma31 is reflected in heterogeneities of clinical trajectories of patients. At this point only sparse information on the correlation between DNA alterations and clinical patterns in multiple myeloma exist.32
Regarding the role of early treatment in the group of patients with indolent multiple myeloma, it is less obvious to form strategies. Borrowing insights from indolent lymphomas,33 early intervention vs delayed intervention (eg, at rising biomarkers) are both options that may be proposed.
Going forward, to me, it seems logical to propose that clinical trials are needed to address the role of early intervention in patients with indolent multiple myeloma. We need properly designed clinical trials to obtain answers in a rational manner. Similar to above, to avoid confusion in the clinical field, before going forward, this will have to be agreed on and endorsed in a consensus document.
Monoclonal gammopathy.
In the 2007 Mayo Clinic study, there is a group of patients with smoldering multiple myeloma who did not progress despite 10 years of follow-up.6 When the pattern of progression for these patients is carefully examined, it is obvious that their rate of progression is superimposed by the rate of progression seen in patients with monoclonal gammopathy of undetermined significance (MGUS) based on the natural history seen in 241 cases.34 In other words, the patients with smoldering multiple myeloma in this category seem to have very similar disease biology as the patients with MGUS. The reason they were labeled as having smoldering multiple myeloma is because they had 10% or more plasma cells in their bone marrow and/or they had M protein of at least 3 g per deciliter in the serum.6 If there were better markers to designate these individuals to the MGUS category (instead of smoldering multiple myeloma), their clinical follow-up would probably have been very different over the time course of 5 to 10 years after initial diagnosis. This dilemma is reflective of many patients who are being followed in clinics all over the world at the current time.
In this context, it is important to briefly review the early original literature on monoclonal gammopathy. To the best of my knowledge, the earliest systematic investigations focusing on abnormal serum proteins started in the 1960s. Several authors35-38 described homogeneous immunoglobulins (paraproteins) in the sera of ageing humans without an underlying B-cell malignancy. Several names were proposed for this condition, including “benign monoclonal gammapathy” and “idiopathic paraproteinemia.” The first clinical and laboratory studies revealed that abnormal proteins occur in about 1% of the adult population, and there is an age-related increase.39-41 These proteins were regarded as essentially benign because the development into multiple myeloma or Waldenström’s macroglobulinemia was observed in only a few cases, despite the large number of cases observed over long periods. The first population-based MGUS screening studies were initiated by Waldenström’s group in Sweden in the early 1960s; a large adult cohort (N = 6995) was screened for pathological serum proteins in 1966.39 In 1978, Kyle published his first retrospective cohort study showing the natural history of 241 cases seen at the Mayo Clinic.34 At that time, the link between abnormal serum proteins and multiple myeloma was still under debate. Some investigators argued that abnormal serum proteins and multiple myeloma were not clearly linked; they felt that “benign monoclonal gammopathy” was the more accurate terminology.35-39 In 1978, Kyle proposed the terminology “undetermined significance” (ie, MGUS), which solved the controversy, and it was adopted broadly.34 From the early 1980s, efforts were made, mainly influenced by Kyle’s group, to establish criteria (discussed in detail above).17
Today, over 50 years after the first studies on this topic were published,35-41 we have gained a lot of additional insights. In 2009, the prospective PLCO cancer screening study, which included over 77 000 participants with up to 10 years of follow-up, identified 71 individuals who stored baseline samples and who subsequently developed multiple myeloma; all of these individuals had detectable abnormal serum proteins in their baseline samples.18 The same patterns were confirmed in a study including stored serum samples from the Department of Defense Serum Repository with cross-reference to an autologous stem cell transplantation database.42
Taken together, prospective screening data have provided definitive evidence that multiple myeloma is consistently preceded by a precursor stage. We have come to a juncture where it is time to acknowledge that monoclonal gammopathy is a precursor condition to multiple myeloma. With new prospective data that link the precursor and multiple myeloma together,18 the “uncertain significance” terminology seems no longer relevant. Indeed, it seems logical to drop the words “uncertain significance” and the acronym “MGUS,” which come from an era when the link between abnormal serum proteins and multiple myeloma was still under debate,34 and, instead, based on new prospective data,18 propose a more accurate terminology: “monoclonal gammopathy” (Table 3).
Going forward, with better markers that more reliably can define patients with low-risk smoldering multiple myeloma, in my opinion, it seems reasonable to propose that this category of patients (ie, all current patients with MGUS and patients with low-risk biology smoldering multiple myeloma), could, in the future, be referred to as 1 entity: “monoclonal gammopathy.” In my opinion, “watch and wait” with longitudinal monitoring should be the default management strategy for this category. Consistent with the above, to avoid confusion in the clinical field, before going forward, this will have to be agreed on and endorsed in a consensus document.
Treating smoldering multiple myeloma: what do we actually know?
First randomized study for patients with smoldering myeloma: in the melphalan era
The first attempt to address the use of active treatment in smoldering multiple myeloma in a randomized study was launched by Hjort and colleagues in 1993.43 In this study, 50 patients with what the authors refer to as “asymptomatic multiple myeloma stage I” were included in a prospective randomized multicenter study (enrollment was between 1983 and 1988) comparing melphalan-prednisone (MP) therapy started at the time of diagnosis with deferred therapy where MP was started at the time of disease progression. The authors randomized 25 patients to each group. The median time from diagnosis to the start of therapy in the group with deferred therapy was 12 months. In the patients with deferred start of therapy, the reasons for starting therapy were increasing M-protein (N = 8), symptomatic bone disease (N = 9), and anemia (N = 5). In 2 cases, disease progression was complicated by vertebral fractures necessitating radiotherapy. The authors report that 2 patients in the group in which MP was started at the time of diagnosis developed acute leukemia. No differences were observed between the 2 treatment groups with regard to response rate, response duration, or overall survival. The authors conclude that in asymptomatic myeloma, deferral of chemotherapy is feasible in well-informed and well-controlled patients but conveys no advantage in overall survival.43
Given the negative results from this first randomized study published in 1993, several review papers have echoed the language from the first cases series by Kyle and Greipp, which stated that “… although chemotherapy may prevent or lessen the complications of active myeloma, therapy may lead to leukopenia, thrombocytopenia, refractory anemia, and acute leukemia. Furthermore, unnecessary chemotherapy causes unnecessary expense, and it is a source of concern to the patient. This form of multiple myeloma, although uncommon, should be recognized, and the patients should be observed without chemotherapy.”4 Several comments could be made about the first randomized study for patients with smoldering myeloma. For example, it had a very small sample size, the therapy was not very efficacious, and there was no focus on patients with high-risk smoldering multiple myeloma; therefore, the results were likely diluted by low-risk/intermediate-risk categories of smoldering myeloma, and the window of enrollment of patients was quite long (5 years), which may have influenced the results.
Randomized study for patients with high-risk smoldering myeloma: using lenalidomide and dexamethasone
Twenty years after the first randomized study for smoldering multiple myeloma43 in 2013, the second randomized study was published by Mateos and colleagues.44 As stated in their paper, the standard of care is observation until symptoms develop; however, this approach does not take into account that patients with high-risk smoldering multiple myeloma may benefit from early intervention. In their randomized, open-label, phase 3 trial, they randomly assigned 119 patients with high-risk smoldering myeloma either to treatment or to observation. Patients in the treatment group received an induction regimen (lenalidomide at a dose of 25 mg per day on days 1 to 21, plus dexamethasone at a dose of 20 mg per day on days 1 to 4 and days 12 to 15, at 4-week intervals for 9 cycles), followed by a maintenance regimen (lenalidomide at a dose of 10 mg per day on days 1 to 21 of each 28-day cycle for 2 years). The primary end point in this study was time to progression to symptomatic disease. Secondary end points were response rate, overall survival, and safety. After a median follow-up of 40 months, the median time to progression was significantly longer in the treatment group than in the observation group (median not reached vs 21 months; hazard ratio for progression, 0.18; P < .001). The 3-year survival rate was also higher in the treatment group (94% vs 80%; hazard ratio for death, 0.31; P = .03). A partial response or better was achieved in 79% of patients in the treatment group after the induction phase and in 90% during the maintenance phase. Toxic effects were mainly grade 2 or lower. Based on these results, the authors conclude that early treatment of patients with high-risk smoldering myeloma delays progression to active disease and increases overall survival.44 In a subsequent follow-up study, the authors reported a median follow-up for surviving patients of 75 months, and lenalidomide plus dexamethasone continued to provide a benefit on time to progression compared to observation (median time to progression not reached vs 23 months; hazard ratio, 0.24; P < .0001).45 Progression to multiple myeloma occurred in 53 (86%) of 62 patients in the observation group compared with 22 (39%) of 57 patients in the treatment group. At data cutoff, 10 (18%) patients had died in the treatment group and 22 (36%) patients had died in the observation group; median overall survival from the time of study entry had not been reached in either group (hazard ratio, 0.43; P = .024). Survival in patients who had received subsequent treatments at the time of progression to active disease did not differ between groups (hazard ratio, 1.34; P = .50).45 The authors concluded that the positive results from their own as well as other ongoing trials support the use of early treatment of patients with high-risk smoldering multiple myeloma in the near future.45
Similar to the first randomized study for smoldering multiple myeloma,43 several comments could be made about this study. For example, the treatment at relapse was not mandated to be lenalidomide and dexamethasone for patients on the observation arm and given drug access in Spain that may have influenced the results; the definition of progression was not the same for the 2 treatment arms (protein increase for the treatment arm and myeloma criteria for the observation arm), which also may have influenced the results. When the progression events in patients who progressed even the first 6 months are looked at, several patients reported bone disease, which raises the question about whether they actually had early (undetected) multiple myeloma.44 Several of these and other comments have been addressed frequently in the literature.
First 3-drug combination therapy for patients with high-risk smoldering myeloma
Inspired by early preliminary results from the (ongoing at the time) second randomized study for patients with high-risk smoldering multiple myeloma using lenalidomide/dexamethasone,44 we explored options to improve efficacy and to preserve safety. Based on our experience with carfilzomib/lenalidomide/dexamethasone therapy in patients newly diagnosed with multiple myeloma (Table 4),14 we hypothesized that we could obtain very deep (ie, molecular) responses to treatment in patients with high-risk smoldering multiple myeloma. Between April 2012 and October 2013, we enrolled 12 patients with high-risk smoldering multiple myeloma on a phase 2 (pilot) trial based on eight 28-day cycles composed of carfilzomib, 20/36 mg/m2 on days 1, 2, 8, 9, 15, and 16; lenalidomide, 25 mg on days 1 through 21; and dexamethasone, 20/10 mg (cycles 1-4/5-8) on days 1, 2, 8, 9, 15, 16, 22, and 23; patients subsequently received 24 cycles of lenalidomide extended dosing. The primary end point was very good partial response or better, and the secondary end point was minimal residual disease (MRD) negativity. We found all patients to achieve a complete response; MRD negativity was found in 11 of 12 (92%) patients by multiparametric flow cytometry and 9 of 12 (75%) patients by next-generation sequencing (Table 4).14 At 12 months of follow-up, all patients remained progression free.14 Although this first pilot study included only 12 patients, as a proof of principle it showed unprecedented deep responses in a high proportion of patients.14 Compared with patients with newly diagnosed multiple myeloma treated with the exact same treatment strategy, also on a phase 2 clinical trial, the results for patients with high-risk smoldering multiple myeloma are strikingly promising (Table 4). These results are highly supportive of future studies investigating the role of active 3- (or 4-) drug combination therapy in combination with MRD testing in patients with high-risk smoldering multiple myeloma. Ongoing studies are investigating the role of active therapy in patients with high-risk smoldering multiple myeloma. Currently, there is a broad range of ongoing studies investigating single-drug (eg, daratumumab), 2-drug (eg, ixazomib/dexamethasone), and 3-drug (eg, carfilzomib/lenalidomide/dexamethasone) combinations (www.clinicaltrials.gov).
To follow up on the promising results from our first 3-drug combination study for smoldering multiple myeloma, we were motivated to prospectively investigate the baseline genetic landscape and patterns of mutations in our cohort of patients. In addition to the 12 patients in the pilot study, we enrolled 6 more patients. As a reference group, we included 40 patients with newly diagnosed multiple myeloma who had received the exact same therapy.14 With a median potential follow-up of 43.3 months, 11 (69%) out 16 evaluable patients with high-risk smoldering multiple myeloma remained MRD negative, and the estimated 4-year progression-free and overall survival in the intention-to-treat population was 71% and 100%, respectively.46 Prior studies with small series of patients have reported comparable mutational burden (ie, median number of mutations) in patients with smoldering multiple myeloma and multiple myeloma.47,48 We confirmed those findings in our follow-up study. Furthermore, we showed that although the number of nonsynonymous mutations was comparable between the 2 groups, there were important differences in the patterns of mutations. Specifically, the frequency of mutations in significantly mutated multiple myeloma genes (6.6% vs 45%) and genes associated with the NF-κB pathway (6.6% vs 25%) were lower in patients with high-risk smoldering myeloma compared with patients with newly diagnosed multiple myeloma.46 The biological underpinnings of these observations remain unknown. Although our study is the largest study to date to assess genetic profiles and clinical outcomes in uniformly treated, using modern 3-drug combination therapy, patients with high-risk smoldering multiple myeloma enrolled in a prospective clinical trial, the sample size precludes definitive conclusions. Larger numbers of patients are needed to further our understanding on this topic.
Summary and future directions: goals of early intervention and how to advance the field?
As indicated in the beginning of this text, the overall scope of this paper is to critically review and dissect data and models on the topics of clinical features, underlying biology, and early treatment trials in smoldering multiple myeloma. The original literature always includes small details that sometimes get lost over time. Hopefully, this literature review makes clear our existing paradigm and helps to facilitate new ideas on how to advance the field.
The observation that high-risk smoldering multiple myeloma seems to benefit from therapy29 and that it can be treated successfully with efficacious 3-drug combinations to obtain unprecedented deep responses (ie, MRD 10−6) in the majority of patients (Table 4) is certainly encouraging.14 However, there are still fundamental questions for which we do not yet have clear answers. For example, for how long does the MRD negativity last? Is it mandatory to remain on maintenance therapy, and what is the optimal duration? Could this be a path toward a cure? These are questions where we, unfortunately, will have to follow patients over time and observe outcomes before we have the answers. If all the patients will relapse eventually, is the 3-drug exposure worth the toxicities, time commitment, costs, etc? Can therapy be stopped after a few years (if so, how many?) and, in case of a recurrence, be resumed with the same efficacy? Instead of using powerful 3-drug therapy,14 is it enough to use more gentle therapy (eg, a 2-drug combination)? If the disease cannot be cured with current drugs, perhaps a long-term low-intensity therapy would be better for (at least some) patients? For these and many more questions we do not have the answers at this time. Clinical trials are needed to address these important questions in a rational manner. Ideally, clinical trials need to include capture and storage of tumor cells and host biology material so that molecular profiling studies can be assessed in relation to treatment response and clinical outcomes.
At the same time as we are facing a lot of complicated questions, to me, there are certain patterns that are quite clear. When the patterns of progression from smoldering multiple myeloma to multiple myeloma in the retrospective Mayo Clinic study are looked at,6 the current definition of smoldering multiple myeloma is a highly heterogeneous clinical entity. As discussed in detail above, in my opinion, there are 3 main clinical patterns (Figure 2): early detection multiple myeloma, indolent multiple myeloma, and monoclonal gammopathy (Table 3). That patients with early detection multiple myeloma, typically, develop multiple myeloma within 1 to 2 years indicates that they have the same disease as patients with multiple myeloma, just at an earlier stage.7,19
In my opinion, the answer to the question raised in the title of this paper (“Shall we treat smoldering multiple myeloma in the near future?”) is yes; we should treat some of them. Specifically, as discussed in this text, it seems logical to propose that patients with high-risk smoldering multiple myeloma should be moved into the definition of multiple myeloma so they can be offered access to active therapy (Table 3). Regarding the role of early treatment in the group of patients with indolent multiple myeloma, it is less obvious to form strategies. Reviewing the literature on indolent lymphomas,33 for example, reveals that early intervention vs delayed intervention (eg, at rising biomarkers) are both options that may be proposed. We need properly designed clinical trials to obtain answers on optimal management of indolent multiple myeloma. Also as discussed in detail above, with new data that link the precursor and multiple myeloma together,18 the “uncertain significance” (ie, MGUS) terminology is no longer relevant. To me, it seems reasonable to propose a new terminology, “monoclonal gammopathy,” which can replace the current MGUS and low-risk smoldering multiple myeloma terminologies (Table 3).
As pointed out in the discussion above, one may always argue that we need more and better data (ie, prospective studies) before it is time to consider changes to the current terminology; however, a counterargument is that such data will take 10 to 20 years to mature, and, indeed, our current clinical terminology is built on the same level of evidence as these proposed ideas.8-12
To caution the reader and to avoid confusion in the clinical field, before going forward, these ideas and concepts will have to be agreed on and endorsed in a consensus document. The intent of this review is to critically review original data and to provide expert opinions based on original data and, in the absence of data, clinical experience beyond the regular text book. The hope is to stimulate the reader to approach the current paradigm in a critical manner and to facilitate avenues for new translational research.
Acknowledgments
The author thanks Memorial Sloan Kettering Cancer Center for support of this work through core grant P30 CA008748.
Correspondence
Ola Landgren, Myeloma Service, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: landgrec@mskcc.org.
References
Competing Interests
Conflict-of-interest disclosure: The author has received research funding from Celgene and has received honoraria from Celgene, Amgen, BMS, Takeda, Merck, Janssen, and Novartis.
Author notes
Off-label drug use: None disclosed.