Abstract
Survivors of allogeneic hematopoietic cell transplantation (HCT) are at risk of developing long-term complications such as subsequent malignancies and cardiopulmonary compromise. The prevalence of chronic health conditions approaches 75% among allogeneic HCT survivors and that for severe or life-threatening conditions exceeds 20%. This chapter describes the burden of morbidity carried by HCT survivors to help healthcare providers and policy makers understand the scope of the problem and the need for life-long follow-up and proactive care for this vulnerable population.
Learning Objectives
To describe the burden of morbidity experienced by allogeneic HCT survivors
To identify populations at increased risk of the long-term complications experienced by allogeneic HCT survivors
To summarize current follow-up recommendations for long-term survivors of allogeneic HCT
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is an established therapeutic option for a variety of hematological malignancies; 80% of those who survive the first 2 years are expected to become long-term survivors.1 However, HCT survivors are at risk of developing long-term complications such as cardiopulmonary compromise, musculoskeletal disorders, endocrinopathies, and subsequent malignancies.2-5 The risk of these complications is influenced by pre-HCT therapeutic exposures, transplantation-related conditioning, and posttransplantation management of GVHD. These complications have a direct impact on the morbidity and mortality experienced by HCT survivors. The burden of morbidity resulting from the cumulative impact of the individual complications occurring after HCT is indeed substantial.6 The cumulative incidence of a chronic health condition among allogeneic HCT survivors is 64% at 10 years and approaches 71% at 15 years after HCT. For severe/life-threatening chronic health conditions, the cumulative incidence approaches 40% at 10 years. The high burden of morbidity experienced by HCT survivors forms the basis for life-long, standardized follow-up of HCT survivors at high risk for post-HCT complications.
This chapter summarizes the magnitude of risk of key long-term complications experienced by HCT survivors, identifying those at increased risk for the complications because of host characteristics and therapeutic exposures. I also describe the health behaviors and patterns of healthcare utilization by the HCT survivors. Finally, I describe the current recommendations for follow-up and monitoring with the goal of early detection such that appropriate management of these complications can result in reduced morbidity.
Cardiovascular disease
Allogeneic HCT survivors are at risk for cardiovascular complications, including arterial disease (cerebrovascular disease and coronary artery disease) and cardiomyopathy. These complications are more common7 and often occur earlier than would be expected in the general population.8 Furthermore, HCT recipients are at a 2-fold increased risk of cardiovascular death compared with the general population.1
Arterial disease
The cumulative incidence of arterial events among allogeneic HCT recipients exceeds 20% at 20 years.8 The median age at first myocardial infarction is 53 years,2 which is much earlier than would be expected for the general population (67 years).9 Arterial disease is primarily due to accelerated atherosclerosis attributed to radiation (pre-HCT and conditioning related) compounded by the cooccurrence of cardiovascular risk factors (CVRFs; hypertension, diabetes, and dyslipidemia).10 Allogeneic HCT recipients are at an increased risk for CVRFs; the 10-year cumulative incidence of hypertension, diabetes, and dyslipidemia is 37.7%, 18.1%, and 46.7%, respectively; the risk for multiple (≥2) CVRFs approaches 40%.2 Conditioning with total body irradiation (TBI) is associated with an increased risk of dyslipidemia and diabetes.2,11 Corticosteroids and calcineurin inhibitors used to manage GVHD also increase the risk of CVRFs.2,12 The already high prevalence of CVRFs in survivors of allogeneic HCT likely accelerates the process of atherogenesis initiated by endothelial injury due to exposure to ionizing radiation and/or chronic GVHD.2,10
Cardiomyopathy
Recommendations for screening, early detection, and prevention
Patients exposed to anthracyclines before HCT should undergo monitoring for late-onset cardiomyopathy using serial echocardiograms. The intensity of echocardiographic screening can range from yearly to every 5 years depending on cumulative anthracycline dose, age at exposure, treatment with mediastinal radiation, and cooccurrence of CVRFs. HCT survivors who received radiation involving the heart field should also be monitored for early-onset atherosclerosis, especially if they have one or more CVRFs. Lifestyles that promote good heart health should be recommended to all survivors, including a regular exercise program, dietary considerations, and screening for (and aggressive management of) dyslipidemia, hypertension, and diabetes. In particular, given the increased risk of dyslipidemia among allogeneic HCT recipients, a lower threshold for management of dyslipidemia is recommended than that practiced in the general community.
Delayed pulmonary complications
Delayed pulmonary complications after allogeneic HCT include the following: bronchiolitis obliterans syndrome (BOS), cryptogenic organizing pneumonia (COP) (formerly called bronchiolitis obliterans with organizing pneumonia [BOOP]), and idiopathic pneumonia syndrome.14,15 These complications usually appear 3 months to 2 years after HCT and are closely related to chronic extensive GVHD. The cumulative incidence of late-onset pulmonary complications is reported to be 10% at 2 years; the cumulative incidence among those diagnosed with chronic GVHD is significantly higher (15.6%). Although pulmonary complications of HCT are often initially subtle, they can progress and become irreversible in the long term and contribute significantly to post-HCT morbidity and mortality.
BOS
BOS is characterized by a nonspecific inflammatory injury affecting the small airways that results in new fixed airflow obstruction. BOS occurs within the first 2 years after HCT, but may develop as late as 4-5 years after transplantation. The prevalence of BOS is 5.5% among all allogeneic HCT recipients and 14% among those who develop chronic GVHD.3 Additional factors associated with increased risk include use of peripheral blood stem cells, exposure to methotrexate for GVHD prophylaxis, older age for recipient and/or donor at HCT, exposure to busulfan for conditioning, prior history of respiratory infection, and low serum immunoglobulin levels. Patients usually present with nonspecific symptoms, including shortness of breath, dry cough, and dyspnea on exertion. Unfortunately, once these symptoms develop, the degree of airflow obstruction is usually already significant and irreversible. Another common manifestation of BOS is the development of air-trapping, which can be appreciated by high-resolution CT scans (persistent lucency of regions of lung parenchyma during expiration, bronchial wall thickening, bronchiectasis, and centrilobular opacities: a mosaic image that carries a sensitivity of 74%–91% and a specificity of 67%–94%16 ). Criteria used to make a clinical diagnosis of bronchiolitis obliterans include: (1) FEV1/FVC <0.7 and FEV1 <75% of predicted value, (2) evidence of air trapping or small airway thickening or bronchiectasis on high resolution CT, and (3) absence of respiratory infection.17 The prognosis of BOS is poor, with reported 5-year survivals of ∼15%. Use of the airflow obstruction classification may permit study of early intervention strategies; a recent consensus conference proposes inhaled corticosteroids and or inhaled bronchodilators for such cases.18
COP
COP is a clinicopathologic syndrome involving the bronchioles, alveolar ducts, and alveoli. Patients with COP are more likely to have GVHD. The syndrome has a cumulative incidence of <2%. It presents as an interstitial pneumonia and usually occurs within the first 12 months after HCT. The clinical presentation in patients is acute, with the sudden onset of a dry cough, dyspnea, and fever. Pulmonary function tests suggest a restrictive pattern and observations from chest x-rays show peripheral patchy consolidation, ground glass attenuation, and nodular opacities. Definitive diagnosis necessitates histologic confirmation.
Idiopathic pneumonia syndrome
Idiopathic pneumonia syndrome usually occurs within the first 4 months after HCT. Risk factors include exposure to TBI and pre-HCT chemotherapy and presence of GVHD and the risk increases with age at HCT. Late-onset interstitial pneumonitis occurs years after HCT, typically in patients with severe chronic GVHD of the sclerodermatous cutaneous variety. A restrictive defect is found on pulmonary function tests.
Recommendations for screening, early detection, and prevention
Monitoring for pulmonary dysfunction in HCT survivors should include the assessment of symptoms such as chronic cough or dyspnea. Because of the insidious onset of BOS and late symptom occurrence, experts advocate pulmonary function testing every 3 months during the first year after HCT. This strategy should allow the capture of patients during a period of tapering of immunosuppression, when most cases of BOS will start to develop, and thus allow an opportunity for earlier intervention. Upon entry into long-term follow-up care, patients should be cautioned about the risks of smoking and exposure to secondhand smoke. It is recommended that pulmonary function tests and chest x-rays are performed upon entry into long-term follow-up for at-risk patients and should be repeated as clinically indicated in symptomatic patients and in those with subclinical abnormalities identified at screening. Influenza and pneumococcal vaccines are recommended in survivors at risk for pulmonary compromise.
Endocrine complications
Endocrine complications are among the most common chronic health conditions encountered after HCT4 ; a brief description of some of them follows.
Thyroid
Thyroid abnormalities include subclinical and overt hypothyroidism.19 Subclinical/compensated hypothyroidism is defined as elevated thyroid-stimulating hormone (TSH) accompanied with normal T4 levels. Conversely, overt hypothyroidism is characterized by low T4 levels accompanied with elevated TSH. The incidence of compensated hypothyroidism ranges from 25% to 30%, with a median latency of 2 years. The cumulative incidence of overt hypothyroidism ranges from 3.4% to 9%, with a latency of 2.7 years. Hypothyroidism is related to radiation to the thyroid gland (neck/mediastinal radiation or TBI). Younger age increases the risk.
Osteopenia/osteoporosis
HCT recipients are at risk for osteopenia and osteoporosis.20-23 The decreased bone mineral density is due to exposure to steroids for GVHD, growth hormone deficiency, hypogonadism, physical inactivity, smoking, excess alcohol intake (risk of falls and poor nutrition), vitamin D deficiency, and a diet low in calcium. The incidence of osteoporosis approaches 20% at 2 years; the most significant loss in bone mineral density occurs during the first 6 months after HCT. Bone mineral loss increases the risk of fractures in the HCT population just as it does in the general population; nontraumatic fractures were observed in 10.6% of the population within 3 years after HCT.
Gonadal failure
Pubertal disturbances after HCT are caused by radiation-related perturbations of the hypothalamic-pituitary axis and/or by chemoradiotherapy-related damage to the gonads.24,25 Testicular germinal epithelium composed of Sertoli cells (responsible for spermatogenesis) is more sensitive to radiation and chemotherapy than the testicular Leydig cell component, which is involved in testosterone secretion. The probability of developing gonadal failure increases with cumulative doses of gonadotoxic therapies. Recovery of spermatogenesis seems to occur more frequently in patients receiving lower doses of cyclophosphamide (120 mg/kg) than in those treated with higher doses (200 mg/kg). Male survivors have a considerable chance of recovering sperm production even when treated with TBI provided that they are <25 years of age at HCT and do not develop chronic GVHD.
Ovaries are more vulnerable to irradiation and chemotherapy.26 Although 50% of prepubertal girls exposed to fractionated TBI enter puberty spontaneously and achieve menarche at a normal age, almost all female patients who are >12 years old when undergoing HCT develop ovarian failure, probably as a result of decreased reserve of primordial follicles. Irreversibility of ovarian function after HCT in most patients highlights the need for timely hormonal replacement to prevent osteoporosis and other complications.
Infertility
Permanent gonadal damage and infertility are known toxicities of preparative regimens used for patients undergoing HCT.27,28 Older age at transplantation, female sex, and exposure to TBI are associated with increased risk for infertility. Infertility has the potential to influence quality of life (QOL); in fact, some survivors have reported that loss of fertility was as painful as confronting cancer. The high prevalence of infertility and the associate distress increases the need for physicians to discuss fertility-preserving options before HCT with prospective recipients and for healthcare providers to provide family-building options and psychosocial support to HCT survivors.
Recommendations for screening, early detection, and prevention
Thyroid.
Screening for thyroid dysfunction relies on a good history and physical examination, as well as annual thyroid function tests (free thyroxine and TSH). Survivors with abnormalities should be referred to an endocrinologist for hormone replacement.
Gonadal function.
Males: Screening for hypogonadism should include an age-appropriate history and Tanner staging with attention to issues related to libido, impotence, or fertility. Measurement of serum luteinizing hormone, follicle-stimulating hormone, and testosterone levels has been recommended as a baseline at age 11 years and in boys whose puberty appears to be delayed. Abnormalities in testicular function should prompt a consultation with the endocrinologist. In females, screening for ovarian dysfunction involves history (primary or secondary amenorrhea, menstrual irregularity, and pregnancies or difficulties in becoming pregnant), Tanner staging, and serum gonadotropin and estradiol levels. For patients with an absence of clinical evidence of puberty, baseline studies should be obtained at the expected time of onset of puberty to assess the need for hormone therapy to induce puberty.
Musculoskeletal complications: osteonecrosis
Osteonecrosis is a debilitating and painful condition that develops when the blood supply to the bone is compromised, usually in areas with terminal circulation.29 It can cause significant morbidity and often requires surgery. The cumulative incidence is 5.4% after allogeneic matched-related donor HCT and 15% after unrelated donor HCT. Males with chronic GVHD and those exposed to calcineurin inhibitors are at increased risk of osteonecrosis.
Recommendations for screening, early detection, and prevention
A careful history and a thorough physical examination are the cornerstone of screening. Early detection could result in the utilization of surgical measures such as cortical decompression, thus either delaying or even obviating the need for joint replacements.
Subsequent malignant neoplasms
An important and potentially devastating complication of HCT is the occurrence of subsequent malignant neoplasms (SMNs). Several host and clinical factors are associated with an increased risk of SMNs after HCT. These include age at HCT, pre-HCT exposure to chemotherapy and radiation, exposure to TBI as part of conditioning, infection with oncogenic viruses (EBV, HBV, and HCV), and prolonged immunosuppression after HCT.5,30-32 It is conventional practice to classify SMNs into 3 distinct groups in the allogeneic HCT setting: (1) lymphoma, including lymphoproliferative disorders; (2) solid tumors; and (3) donor-derived acute leukemia.
Hodgkin lymphoma
Hodgkin lymphoma is observed after HCT.33 The most common subtype is mixed cellularity and most of the cases contain the EBV genome. These cases differ from the EBV posttransplantation lymphoproliferative disorder by the absence of risk factors commonly associated with that disorder, by a later onset (>2.5 years), and by a relatively good prognosis.
Solid tumors
The cumulative incidence of solid tumors after allogeneic HCT ranges from 7% to 11% at 15 years after transplantation.5,30-32 The magnitude of risk of solid tumors exceeds 2-fold that of an age- and sex-matched general population; the risk reaches 3-fold among patients followed for 15 or more years after HCT. Although radiation (both pre-HCT exposure and TBI) are associated with an increased risk of solid tumors, several other factors (host and clinical) increase the risk of specific solid tumors. These include infection with oncogenic viruses (HBV, HCV: hepatocellular carcinoma; HPV: cervical cancer), chronic GVHD of the oral cavity (squamous cell carcinoma of the oral cavity), and exposure to busulfan in smokers (lung cancer). The association between solid tumor radiation therapy used to treat the primary cancer is typically associated with a long latency and the risk is high among those exposed to irradiation at a young age. Therefore, among patients exposed to radiation at <30 years of age, the risk is 9-fold that of the general population, whereas for those >30 years, it approaches that of the general population. Solid tumors commonly seen after HCT include melanoma and cancers of the oral cavity and salivary glands, brain, liver, cervix, thyroid, breast, bone, and connective tissue.
Skin cancer
Among allogeneic HCT recipients, the incidence of basal cell carcinoma is 6.5% at 20 years and that for squamous cell carcinoma is 3.4%.34 TBI increases the risk of basal cell carcinoma, especially in the younger patients. Squamous cell carcinoma risk is increased among patients with acute GVHD, whereas chronic GVHD is associated with both basal cell carcinoma and squamous cell carcinoma. In patients with prolonged immunosuppression, oncogenic viruses such as HPV contribute to squamous cell carcinoma of the skin and buccal mucosa.
Breast cancer
The 25-year cumulative incidence of breast cancer is 11% after allogeneic HCT,35 conferring on these survivors a 2.2-fold increased risk of developing breast cancer compared with the age- and sex-matched general population. The median latency from HCT to diagnosis of breast cancer is 12.5 years. The incidence is higher among those exposed to TBI (17%) than among those who did not receive TBI (3%). The risk is increased among those exposed to TBI at a younger age.
Thyroid cancer
HCT recipients are at a 3.3-fold increased risk of thyroid cancer compared with the age- and sex-matched general population.36 Age younger than 10 years at HCT, neck radiation, female sex, and chronic GVHD are associated with an increased risk of thyroid cancer. Thyroid cancer develops after a latency of 8.5 years and is associated with an excellent outcome.
Colorectal cancer
Recent reports indicate an association between abdominal radiation and colorectal cancer. These reports have emerged primarily from childhood cancer survivor cohorts treated with conventional therapy, but warrant a discussion here for HCT recipients exposed before HCT with abdominal radiation. The Childhood Cancer Survivor Study demonstrated an 8.5-fold increased risk of colorectal cancer among patients exposed to abdominal radiation compared with the general population.37 In a large cohort of patients treated at French and British centers, Tukenova et al demonstrated a strong dose-response relationship with radiation exposure; the odds of developing a gastrointestinal malignancy were 5.2-fold increased for patients treated with abdominal radiation between 10 and 29 Gy compared with those not exposed to radiation; the odds were 9.6-fold increased among those treated with radiation doses exceeding 30 Gy.38
Recommendations for screening, early detection, and prevention
Close monitoring is important for those at risk. Screening recommendations for radiation-related second cancers include careful physical examination of the skin and underlying tissues in the radiation field. Because outcome after treatment for breast cancer is closely linked to stage at diagnosis, close surveillance to promote early diagnosis should confer survival advantage. The Children's Oncology Group (COG) recommendations39 for those at risk (ie, radiation doses of 20 Gy or higher to the mantle, mediastinal, whole lung, and axillary fields) include: monthly self-examination of the breast beginning at puberty annual clinical breast examinations beginning at puberty until age 25 years, and clinical breast examinations every 6 months, with annual mammograms and MRIs beginning 8 years after radiation or at age 25 (whichever occurs later). Screening of those at risk for early-onset colorectal cancer (ie, radiation doses of 30 Gy or higher to the abdomen, pelvis, or spine) should include colonoscopy every 5 years beginning at age 35 years or 10 years after radiation (whichever occurs last).
QOL after HCT
Several studies have examined post-HCT recovery and QOL.40-43 The majority demonstrate that physical QOL is stable from pre-HCT to post-HCT; psychological, social, and spiritual QOL improved at 6 months. Older patients report worse physical but better social well-being. Vulnerable populations are determined by the presence of chronic GVHD. The ability to hold a job is an important indicator of rehabilitation to normal life. Approximately 2/3 of HCT patients return to work by 1 year and 75% return to work by 2 years; the overwhelmingly major reason for not returning to work was related to poor health, primarily due to the presence of active chronic GVHD.
Late mortality
The high burden of morbidity carried by HCT recipients can result in premature death. The conditional survival probability at 10-20 years after HCT exceeds 80% for those individuals who were alive and disease-free 2-5 years after HCT.1,44 Mortality rates were 4-fold to 9-fold higher than in the expected population for at least 30 years after HCT, producing an estimated lower life expectancy of 30% compared with the general population regardless of current age.45 Relapse of primary disease and chronic GVHD are the main causes of premature death. Non-relapse-related mortality is greater among patients who are >18 years of age when undergoing HCT, as well as among those with chronic GVHD. Compared with the general population, allogeneic HCT recipients are 3.6 times more likely to die of SMNs, 15.1 times more likely to die of pulmonary complications, and 2.3 times more likely to die of a cardiac compromise.
Health behaviors of long-term survivors of HCT
The high burden of morbidity carried by HCT survivors necessitates that they adopt healthy lifestyles and develop strategies in their daily lives that incorporate health promotion, prevention, and surveillance for early detection of complications. Reports find that HCT survivors have comparable cervical and testicular cancer screening practices, are more likely to have had breast cancer screening by mammography, and are less likely to be engaged in high-risk behaviors compared with healthy sibling controls.46,47 Furthermore, HCT survivors are less likely to indulge in high-risk behavior (smoking and excessive alcohol intake) than their siblings. However, despite potential long-term risks, certain subsets of survivors (younger patients and those with lower education) continue to engage in high-risk behaviors such as smoking and excessive alcohol intake, indicating the need for targeted interventions for these high-risk populations. Continued vigilance in encouraging appropriate cancer screening and healthy behaviors for HCT survivors is critical.
Healthcare utilization by HCT survivors
In a survey of 10+ year HCT survivors, whereas >99% maintained some level of medical contact, only 25% returned to the transplantation center for management of ongoing health issues in the 2 years before study participation.48 This could be because of distance from the center, official transition of care by the transplanting team to the primary care physician, or the expiration of the contractual arrangement between the patients' insurance carrier and the transplantation center. The observation that a large proportion of allogeneic HCT recipients do not return to the transplantation center for long-term care (despite the high burden of morbidity) emphasizes the need for ensuring that standardized recommendations for the follow-up of this vulnerable population are disseminated to the primary care physicians assuming responsibility for the care of these patients. Furthermore, there is a need for the transplantation team to develop an ongoing partnership with the primary care physicians as patients are transitioned from the transplantation center to ensure that comprehensive care is delivered long-term to the survivors.
Long-term follow-up guidelines
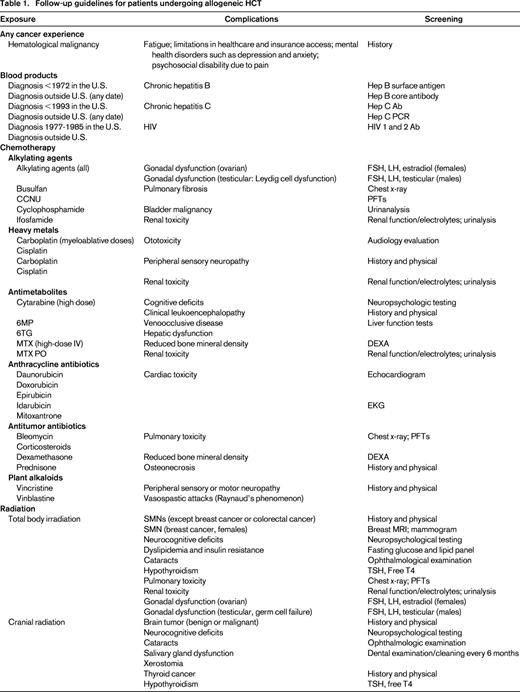
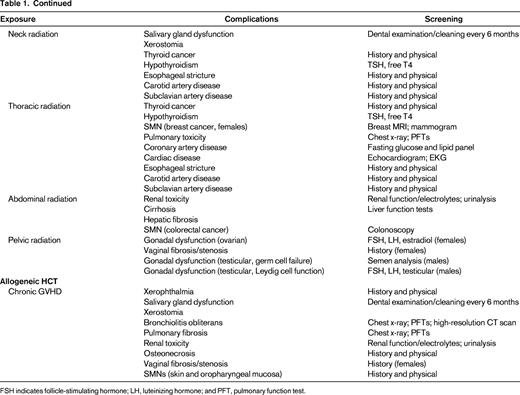
Theoretically, if screening resulted in an earlier diagnosis of key complications, then management of these complications would be more successful. The issue of whom to screen, how to screen, and when to do it are all important but largely unanswered questions. For HCT survivors, screening recommendations must incorporate risks associated with pre-HCT therapy, transplantation conditioning therapy, and other HCT-associated conditions such as chronic GVHD. The Center for International Blood and Marrow Transplantation Research (CIBMTR), the European Group for Blood and Marrow Transplantation (EBMT), and the American Society for Bone Marrow Transplantation (ASBMT) have developed recommendations for screening and early detection of complications after HCT.49 These recommendations concentrate on risks faced by patients beyond 6 months after transplantation. Most of these recommendations are derived from studies that have identified specific complications in long-term survivors and the risk factors associated with them. The guidelines are organized by organ system and time after HCT; details include potential late effects, known risk factors, and recommendations for tests and preventive measures.
For the pediatric survivor population, COG has developed comprehensive long-term follow-up guidelines that provide risk-adapted yet consensus-based recommendations.39 The overall goal is early identification of treatment-related complications, potentially allowing for early intervention with a resultant reduction in morbidity and mortality and an attendant reduction in healthcare costs. Because treatment exposures vary by primary diagnosis, HCT type, patient age, and treatment era, a therapy-based approach was chosen. These guidelines have been developed as a resource for clinicians who provide ongoing healthcare to HCT survivors. The COG guidelines are intended for use beginning 2 or more years after completion of therapy and provide a framework for monitoring of late effects in survivors. The recommendations for periodic screening evaluations provided in these guidelines are appropriate for asymptomatic survivors who present for routine medical follow-up. Therefore, unlike the EBMT/CIBMTR/ASBMT guidelines, the COG guidelines utilize the cumulative therapeutic exposures to generate a tailored list of potential late effects and the recommended screening for early detection of those late effects. The intensity of screening is determined by whether the patient is at standard risk or increased risk due to cumulative therapeutic exposures and/or host/demographic characteristics. This provides a greater level of granularity and specificity to the screening that the patient undergoes and therefore eliminates the possibility of overscreening or underscreening.
In summary, each patient should receive a summary of their therapeutic exposures. These should include exposures experienced before and at the time of HCT, as well as their GVHD status. These exposures should then inform the screening recommendations and follow-up care of the patient. Figure 1 serves as an example of the template for therapeutic exposure summary for the patients. Table 1 suggests some of the screening recommendations drawn from the guidelines.
Risk-reduction strategies
Although strategies aimed at altering therapeutic exposures (chemotherapy and radiation) will most likely result in a reduction in the risk of complications, it is unlikely that these exposures are the only factors that convey risk. Furthermore, it is often not feasible to alter therapeutic exposures while preserving clinical outcomes. There is increasing concern related to the risk of cancer and cancer death associated with obesity and a sedentary lifestyle.50 Functional and performance limitations and lower levels of physical activity have been reported in HCT survivors, particularly in those who have chronic GVHD.51 Obesity (as determined by body mass index) is uncommon in HCT survivors11 ; however, preliminary studies of body composition reveal that they have a high percentage of fat mass and a reduction in muscle mass. This state of sarcopenic obesity contributes to the development insulin resistance, hyperinsulinemia, and chronic inflammation, all factors that have been implicated in the causal pathway of obesity-associated cancer risk and cardiovascular complications. Therefore, interventions recommended for the general population aimed at improving nutrition and maintaining a physically active lifestyle are likely of even greater significance for HCT survivors as risk-reduction strategies. Finally, as the pathogenesis of the complications after HCT becomes more clearly elucidated, screening for known genetic susceptibility to these complications could possibly be undertaken and alternative therapeutic options presented to the vulnerable subpopulation. These strategies, as well as those encouraged for all, such as avoidance of tobacco and alcohol, use of sunscreen, and HPV vaccination, may help to reduce the risk of complications in HCT survivors.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (Grant R01 CA078938 to S.B.) and the Leukemia & Lymphoma Society (Grant 2192 to S.B.).
Disclosures
Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
Correspondence
S. Bhatia, City of Hope National Medical Center, 1500 East Duarte Road, Duarte, CA 91010-3000; Phone: (626)471-7321; Fax: (626)301-8983; e-mail: sbhatia@coh.org.