Abstract
Hemophagocytic lymphohistiocytosis (HLH) is not an independent disease but rather a life-threatening clinical syndrome that occurs in many underlying conditions and in all age groups. HLH is the consequence of a severe, uncontrolled hyperinflammatory reaction that in most cases is triggered by an infectious agent. Persistent stimulation of lymphocytes and histiocytes results in hypercytokinemia, leading to the characteristic symptoms of HLH. Genetic defects in familial HLH and in immunodeficiency syndromes associated with albinism affect the transport, processing, and function of cytotoxic granules in natural killer cells and cytotoxic T lymphocytes. This leads to defective killing of target cells and a failure to contract the immune response. The defects are increasingly found also in adolescents and adults. Acquired HLH occurs in autoinflammatory and autoimmune diseases (macrophage activation syndrome) and in patients with iatrogenic immunosuppression or with malignancies, but also in otherwise healthy persons with infections. Treatment of HLH aims at suppressing hypercytokinemia and eliminating the activated and infected cells. In genetic HLH, hematopoietic stem cell transplantation (HSCT) is needed for the correction of the immune defect. Treatment modalities include immunosuppressive, immunomodulatory, and cytostatic drugs; T-cell antibodies; and anticytokine agents. Using immunochemotherapy, familial HLH, which had been invariably fatal, has become a curable disease with more than 50% survivors. Reduced intensity conditioning for HSCT, which is associated with less transplantation-related mortality, will further improve cure rates.
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a hyperinflammatory syndrome with high mortality even with appropriate treatment. This condition, which shares features with sepsis and systemic inflammatory response syndrome (SIRS), has received increasing attention in recent years such that 85% of the 1500 or so publications on HLH have appeared in the last decade.
HLH is a clinical syndrome
The definition of HLH is the key to understanding HLH: HLH is not a single disease, but rather a hyperinflammatory syndrome caused by excessive activation of lymphocytes and macrophages that produce high levels of cytokines. HLH is associated with many underlying conditions and affects all age groups. The cardinal symptoms and signs of HLH are prolonged high fever, hepatosplenomegaly, and cytopenias. Characteristic laboratory abnormalities include elevated ferritin, triglycerides, transaminases, bilirubin, and lactate dehydrogenase and low fibrinogen. Hemophagocytosis is not an obligatory symptom and may be absent initially.
Recently, attention has been drawn to atypical symptoms in genetic HLH, including chronic diarrhea, sensorineural hearing loss, and a clinical presentation resembling chronic variable immunodeficiency.1,2 Manifestation of HLH exclusively or predominantly in one organ, such as the central nervous system (CNS) or liver, may present diagnostic problems.
All symptoms and laboratory changes can also be found to some extent in patients who can cope with an infectious trigger by mounting an adequate and effective inflammatory response. HLH represents the extreme end of the spectrum of inflammatory reactions and is characterized by the magnitude of the clinical and laboratory abnormalities and the progressiveness of the symptoms.3 When hyperinflammation cannot be contained, patients die from multiorgan failure, CNS dysfunction, and bacterial and fungal infections due to prolonged neutropenia. For more detailed information, the reader is referred to recent reviews.4-6
Genetic and acquired HLH
Until recently, age was thought to roughly discriminate between genetic (“inherited”) and acquired (“secondary”) forms of HLH. However, the detection of an increasing number of genetic cases in adolescents and adults proves this assumption erroneous.1,7 Specifically, in a recent study on 175 adult patients with HLH, hypomorphic monoallelic or biallelic mutations in genes of familial HLH (FHL) were found in 14% of the patients.7
Four genetic defects have been identified in FHL and all of the genes are involved in cytotoxic granule exocytosis or function (Table 1). In Germany, less than 10% of patients with FHL show none of the 4 gene defects. Familiarity in these patients can be assumed by an abnormal degranulation test or persistently deficient activity of natural killer (NK) cells (see “How to differentiate genetic HLH from acquired HLH” below).
Griscelli syndrome 2 (GS-2) and Chédiak-Higashi syndrome (CHS) are immunodeficiency syndromes characterized by albinism, neutrophil dysfunction, recurrent infections, and, in CHS, neurological dysfunctions. They present frequently with HLH either as first manifestation of disease or later during the course of disease. As in FHL-3-5, processing of cytotoxic vesicles is impaired; in addition, there is a defective release of melanin from melanosomes. Neutrophil and platelet function also depends on trafficking and exocytosis of cell-specific vesicles that are mediated by proteins that are important for cytotoxic granule processing.
Acquired HLH is associated with a variety of underlying conditions, among which infection-associated HLH is the most common form (Table 1). Herpes viruses, especially EBV, are the leading triggers. Interestingly, whereas in infectious mononucleosis, EBV infects B cells, in EBV-associated HLH, the virus is found either predominantly in T cells (in cases from Asia8 ) or in equal proportions in B and T cells (in white children9 ). Nearly every other infectious organism, especially viruses but also bacteria, fungi, and protozoa, have been implicated in HLH. In our database, Leishmania is the second most frequent infectious trigger after EBV.
Macrophage activation syndrome (MAS) is a variant of HLH that occurs in patients with autoinflammatory or autoimmune diseases. Most MAS cases have been described in systemic juvenile idiopathic arthritis (sJIA) and its adult-onset form, but also in systemic lupus erythematosus or other rheumatic diseases.10 MAS can be the presenting feature of sJIA, rendering the distinction from classical HLH difficult, especially when arthritis is absent initially. The close relationship of MAS in sJIA to other forms of HLH is supported by several lines of evidence, such as decreased NK cell function and perforin expression and association with single nucleotide polymorphisms in UNC13D and PFR1 in children with MAS.11
Diagnosis of HLH
A prerequisite for diagnosis is the awareness that a seemingly normal infection may evolve into the inadequate hyperinflammatory response that characterizes HLH. Timely diagnosis is critical to start therapy before damage by hypercytokinemia becomes irreversible. There is no single feature that is specific for HLH, including hemophagocytosis, but the triad of prolonged fever, hepatosplenomegaly, and cytopenias should arouse suspicion of the possibility of HLH.
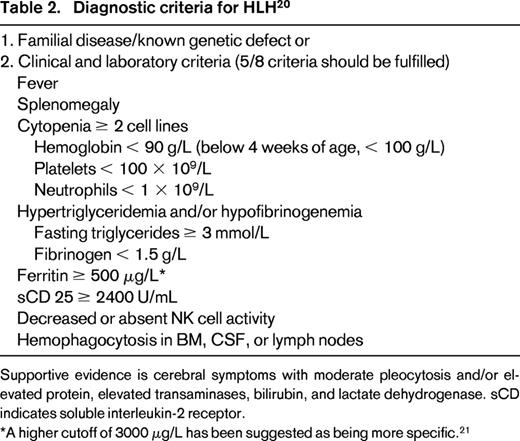
To establish a common basis for treatment, the HLH Study Group of the Histiocyte Society has proposed diagnostic criteria for HLH that have been revised recently20 (Table 2). Frequently, diagnostic criteria are not yet fulfilled at initial presentation.3 A higher cutoff for ferritin might be better, as suggested by some investigators, who found values of 3000 μg/L or 10 000 μg/L to be more helpful.21,22 In patients with MAS, HLH 2004 criteria are not appropriate because they are fulfilled only at a late stage. Presently, an international collaboration aims at developing new diagnostic criteria for MAS complicating sJIA.11
How to differentiate genetic HLH from acquired HLH
It should be emphasized that severity of the disease and not classification (genetic or acquired) should govern initial treatment. However, information about an underlying genetic defect is important for management because it will allow for an early search for a stem cell donor. Severity of disease and the identification of an infectious agent do not discriminate between genetic and acquired forms of HLH. Age is helpful to some extent: a minority of children < 1 year of age will have acquired HLH, but older age does not reliably exclude genetic HLH.
Impaired NK cell cytotoxicity is a characteristic finding in FHL and immunodeficiency syndromes with albinism; however, normal activity does not exclude either. Moreover, decreased function of NK cells has been also observed in patients with acquired HLH, in patients with MAS, and in close relatives of patients with FHL. However, NK cell activity is still a valuable tool to confirm findings of other assays.
Recently, flow cytometry has been used as a screening method to identify patients with genetic predisposition to HLH.23 Intracellular stains are available that measure perforin, SAP (x-linked lymphoproliferative syndrome [XLP-1]), and XIAP (XLP-2). Munc13-4 protein expression in platelets has been reported as potential new rapid screen for FHL-324 and is awaiting testing in a larger cohort. Genetic defects with impaired granule exocytosis (FHL 3-5, CHS, and GS-2) lead to impaired translocation of the lysosome-associated membrane glycoprotein CD107a to the cell surface upon stimulation of NK cells or cytotoxic T lymphocytes (CTLs). In 494 patients evaluated within a collaborative European study, the NK degranulation assay clearly discriminated between patients with defects in granule exocytosis and patients with acquired HLH or other hereditary defects such as perforin, SAP, or XIAP deficiency.25 Once these functional tests suggest a genetic basis for HLH, molecular analysis should follow, including for parents and siblings.
Lately, some parameters have been suggested to facilitate distinction between MAS and classical HLH and between MAS and flares of sJIA. Comparing patients with MAS with patients with FHL and infection-associated HLH, neutrophils > 1.8 × 109/L, C-reactive protein > 90 mg/L, and soluble CD25 < 7900 U/mL were indicative of MAS with a high sensitivity and specificity.26 The dynamics of leukocytes, platelets, and fibrinogen are helpful to distinguish MAS from exacerbations of sJIA.11,23
Pathogenesis
HLH represents a hyperinflammatory uncontrolled immune response triggered by various stimuli. Much has been learned about the pathogenesis of genetic HLH, whereas the mechanisms leading to acquired HLH are still poorly understood.
Triggers in HLH
Five murine HLH models are available that display the same disease manifestation as humans: Perforin, UNC13D, STX11, RAB27a, and LYST. All mouse models require a viral trigger to develop HLH. CD8+ T cells secreting high levels of IFNγ play a major role in disease pathogenesis.27,28 Recently, the mild course of HLH in syntaxin11-deficient mice was shown to be due to T-cell exhaustion.28
In patients, infectious triggers cannot always be identified; however, pathogens may be missed if extensive microbiological studies are not performed. Non-antigen-specific triggering is another mechanism leading to HLH. Cells of the innate immune response are activated via pattern recognition receptors. These are, among others, toll-like receptors (TLRs) that respond to components of bacteria, mycoplasma, fungi, and viruses.29 Patients with bacterial sepsis or with systemic inflammatory response syndrome (SIRS) may develop a clinical picture with all the characteristic features of HLH, including hemophagocytosis and decreased NK cell activity.30 TLR signaling may also play a role in genetic HLH, as shown in the UNC13Djinx/jinx mouse, an animal model of FHL-3: inactivation of the TLR adaptor protein MyD88 in LCMV-infected mice reduced CD8+ T-cell expansion and macrophage activation.31 Recently, it has been shown that repeated TLR9 stimulation in genetically normal mice leads to an MAS-like disease.32 Accordingly, gene expression profiles in sJIA patients revealed up-regulation of genes triggered by TLR/IL-1R signaling.33 It has been suggested that intrinsic hyperactivity of these genes may lead to MAS in patients with sJIA.32 However, MAS may also be triggered by infectious agents. HLH in several patients with metabolic diseases such as lysinuric protein intolerance and lipid storage diseases suggests that metabolic products may also serve as trigger for immunostimulation.
Symptoms of HLH reflect hypercytokinemia
Activated lymphocytes and macrophages secrete high levels of pro- and anti-inflammatory cytokines and chemokines, which leads to the characteristic clinical and laboratory findings.3 Fever is caused by interleukins and TNFα. Ferritin is secreted by activated macrophages, which also produce increased levels of plasminogen activator, leading to hyperfibrinolysis. Cytokines suppress lipoprotein lipase and hematopoiesis. Hemophagocytosis is probably not the only factor accounting for the profound cytopenias. Interestingly, cytokine-mediated down-regulation of CD47, which prevents phagocytosis by interaction with the signal regulatory protein α (SIRPA), has been described recently in the hematopoietic stem cells of patients with HLH.34 This could explain the evolution to BM aplasia in patients with poorly controlled disease.
Defective cytotoxic function is essential in genetic HLH
In immunocompetent individuals, NK cells and CTLs kill infected cells by a nonsecretory pathway involving Fas ligand (CD95-L) but, more importantly, by a perforin-dependent pathway. Cytotoxic cells are equipped with cytotoxic granules, also called secretory lysosomes, which contain perforin and granzymes. Upon activation of NK cells or CTLs, these granules are carried along microtubules toward the immunological synapse between effector and target cell. In this complex process, granules have to be activated to migrate, dock, and fuse with the cell membrane and to release their contents into the synapse.35 Together with granzymes, perforin then mediates apoptotic death of target cells and the immune response is down-regulated.
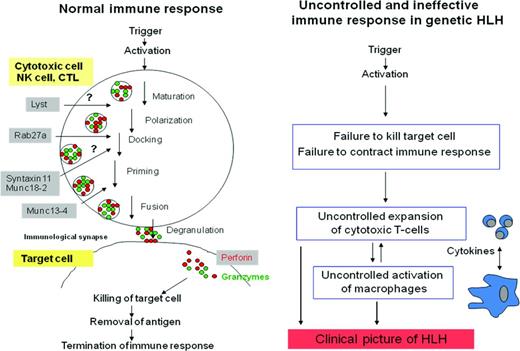
Patients with FHL-2 have mutations leading to reduced or absent perforin. Mutated genes in FHL3-5 and in GS-2 and CHS are involved in cytotoxic granule processing during various steps of trafficking and exocytosis.36 Therefore, the pathogenesis of genetic HLH is very likely based on the inability of cytotoxic cells to kill and eliminate the infected antigen-presenting cell (APC; Figure 1).
Immune response in healthy subjects and uncontrolled, ineffective immune response in patients with genetic HLH.6 Perforin and granzymes are secreted via cytotoxic granules, leading to apoptosis of the target cell. Processing of cytotoxic granules requires several steps, including polarization, docking, priming, and fusion with the cell membrane. Genes mutated in FHL-3, FHL-4, and FHL-5 and in the immunodeficiency syndromes CHS and GS-2 encode proteins that are crucial for these processes.
Immune response in healthy subjects and uncontrolled, ineffective immune response in patients with genetic HLH.6 Perforin and granzymes are secreted via cytotoxic granules, leading to apoptosis of the target cell. Processing of cytotoxic granules requires several steps, including polarization, docking, priming, and fusion with the cell membrane. Genes mutated in FHL-3, FHL-4, and FHL-5 and in the immunodeficiency syndromes CHS and GS-2 encode proteins that are crucial for these processes.
In patients with XLP-1, lymphocyte cytotoxicity is also affected; in patients with XLP-2, IL-2-inducible T-cell kinase (ITK), and CD27 deficiency, the molecular mechanisms are largely unknown.
Failure of down-regulation of the immune response in FHL
In healthy persons, immune homeostasis is maintained by contraction of the immune response after successful elimination of the trigger. Perforin plays a critical role in this process. In mice, control of the immune response was shown to involve perforin- and Fas-dependent killing of APCs (dendritic cells) by CTLs37 and regulation of antiviral CTL response by perforin-dependent killing of activated CD4 cells by NK cells.38 Lysis of dendritic cells by NK cells has also been shown to occur in humans. Failure to kill APCs would not only impede reduction of the viral load, but also result in continuous stimulation of immune cells. “Fratricide” has been proposed as another mechanism of down-regulation of the immune response, where CTLs that have captured membrane fragments and antigenic peptides from the target cell have become susceptible to lysis by other CTLs.39 Finally, it has been suggested that failure of cytotoxic CD4+ T-regulatory cells to kill the largely expanded CD8+ T-cell population may contribute to the pathology of HLH.40 It remains to be shown which mechanisms are crucial in HLH.
Possible mechanisms in acquired HLH
Mechanisms in acquired HLH are probably diverse and may have to act in combination. Some patients with acquired HLH are immunosuppressed, which may result in the inability to cope with an infectious trigger, thus leading to HLH. As discussed above, in patients with autoinflammatory and autoimmune diseases, non-antigen-specific stimulation of innate immunity may be the trigger for HLH. Direct activation of TLRs by intracellular pathogens that persist in histiocytes may explain the fact that many cases of infection-associated HLH have been described with Leishmania, Mycobacterium tuberculosis, or Salmonella typhimurium. However, this leaves a large number of cases without a good explanation. Some of these patients may have hypomorphic mono- or biallelic mutations in FHL genes.7 Other mechanisms may involve single nucleotide polymorphisms (SNPs) in genes important in the immune response and imbalance between infected cells and immune effector cells. In fact, many patients with acquired HLH have low NK cell numbers. Finally, immune evasion strategies by viruses can interfere with NK and CTL cytotoxicity.
Treatment
General considerations
The optimal treatment of HLH is not known and varies depending on the underlying disease and severity of symptoms. Treating HLH is challenging. Hyperinflammation has to be suppressed to prevent or treat the deleterious effects of hypercytokinemia, including coagulopathy, prolonged neutropenia, CNS hyperinflammation, and impending organ failure. At the same time, immunosuppressive and cytostatic treatment may interfere with the patient's defense mechanisms needed to successfully contain an infectious agent. Immune defense may already be compromised in many patients with HLH; however, it could be worsened by disturbances in other pathways of the immune system, delayed BM recovery, or afflicted defense barriers such as skin and mucous membranes. Prolonged immunosuppressive treatment could not only lead to reactivation of the original trigger and of other dormant infectious agents, but also to an increased susceptibility toward a new triggering agent. Therefore, judicious use of available therapeutic agents is important. Assuming that the infection has to be treated first and only then HLH is a dangerous misconception.
It is controversial whether patients with sepsis who develop the clinical picture of HLH should be treated according to HLH protocols.30 It is self-evident that appropriate antibiotic treatment and supportive care are the most important measures. However, it is our opinion that an additional short course of corticosteroids and /or IV immunoglobulin treatment to control hypercytokinemia may be beneficial when there is incipient organ failure. In later stages of sepsis/SIRS, when the combined expression of pro- and anti-inflammatory molecules leads to apoptosis of cells of the innate and adaptive immune system and to impaired host immunity,41 corticosteroids may be counterproductive. Cytostatic drugs should be avoided completely.
Principles of treatment (Table 3)
Although suppression of hyperinflammation usually requires immediate action and should not be postponed, the search for a treatable trigger is mandatory. Therapy of an infectious agent does not render anti-inflammatory treatment unnecessary (except in Leishmania-associated HLH), but may contribute to a faster reduction of the antigenic burden. Corticosteroids are the most important anti-inflammatory drugs for HLH. Due to its better penetration into the CSF, dexamethasone may be superior. Less severe cases may do well with corticosteroids and immunomodulatory drugs such as cyclosporine A (CSA) or immunoglobulins; however, these patients have to be followed carefully. In MAS, a pulse of high-dose corticosteroids with or without CSA is effective in most patients. Lately anticytokine treatment has been used successfully.
Eliminating activated lymphocytes and (infected) APCs is another goal of treatment. Etoposide is an effective agent for monocytic and histiocytic diseases. The 2 HLH study protocols of the Histiocyte Society have used a combination of dexamethasone, etoposide, and CSA, followed by HSCT for familial disease.20,42 The estimated 5-year probability of survival in the HLH-1994 study was 54%. Failure was due to nonresponse/reactivation before HSCT or to transplantation-related mortality.42 In patients with EBV-associated HLH, the addition of rituximab seems to be a valuable adjunct to therapy. However, it has to be remembered that, in contrast to a normal infection with EBV, in HLH patients, the virus is also incorporated in T cells.9
Antithymocyte globulin was used successfully in a monocentric study.43 Alemtuzumab, an antibody against CD52 that is present not only on T cells but also on histiocytes, has been shown to be beneficial in patients with refractory HLH.44 Plasma exchange, a historical treatment for FHL, may still be of use for patients who do not respond to standard treatment.45
A promising approach is to target the high IFNγ levels; in 2 different mouse models, this approach was successful in controlling HLH.46 A clinical study in relapsed/refractory patients with an IFNγ antibody is under way.
In patients with genetic HLH, HSCT has to be performed to correct the immune defect. Results are equal with matched related or unrelated transplantations.47 Increased mortality with myeloablative conditioning has prompted the use of less toxic approaches. Recently, reduced-intensity conditioning was shown to be associated with lower toxicity and increased survival.48 Mixed chimerism is more frequent after reduced-intensity conditioning; however, murine studies and clinical observations suggest that a stable chimerism with 10%-20% donor cells may be sufficient.49 A recent survey confirmed the benefit of HSCT in patients with XLP-1.50 The indication for patients with XLP-2 should be based on the severity of the clinical course.
Conclusion
HLH is a dangerous hyperinflammatory syndrome with highly characteristic, but nonspecific, symptoms and laboratory findings. A high level of awareness is necessary to consider HLH in patients with prolonged fever, hepatosplenomegaly, and cytopenias. Genetic causes identified to date affect cytotoxic function of NK and cytotoxic T cells. Hypomorphic mutations in these genes are increasingly found in adolescents and adults. Functional tests quickly differentiate genetic from acquired HLH before results of genetic tests are available. Treatment of HLH that targets the activated lymphocytes and histiocytes remains a challenge. It can be life-saving but may interfere with remaining immune functions. The use of reduced-intensity conditioning regimens for HSCT has been a big step forward in that the high treatment mortality after HSCT with myeloablative conditioning has been reduced dramatically.
Disclosures
Conflict-of-interest disclosure: The authors declare no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Gritta Janka, Klinik und Poliklinik für Pädiatrische Hämatologie und Onkologie, Universitätsklinikum Hamburg-Eppendorf, Martinistr 52, Hamburg 20246, Germany; Phone: 49-40-741054369; Fax: 49-40-741058250; e-mail: janka@uke.uni-hamburg.de.