Abstract
A 6-month-old girl was diagnosed with acute lymphoblastic leukemia (ALL). She has completed induction therapy and is currently in first complete remission (CR1). You are asked by your resident if hematopoietic stem cell transplantation (HSCT) would benefit infants with acute leukemia.
Advances in risk stratification, intensification of therapy, and progress in supportive care have all led to improved outcomes in pediatric ALL and acute myeloid leukemia (AML). Cure rates now approach 85% in pediatric ALL and 60% in pediatric AML.1 However, infants with ALL, especially those less than 6 months of age at diagnosis and those with rearrangements of the MLL gene, have the poorest outcomes, with cure rates of < 50%.2 MLL rearrangements are associated with intermediate to poor prognosis in both ALL and AML.1,3 Further, MLL rearrangements are most common in infants, with MLL rearrangements being present in ∼ 70% to 80% of cases of infant ALL and in 50% to 60% of cases of infant AML.4,5 To improve outcomes for infants with leukemia, recent trials have intensified therapy and several studies have explored the use of HSCT in CR1 in infant ALL and AML.
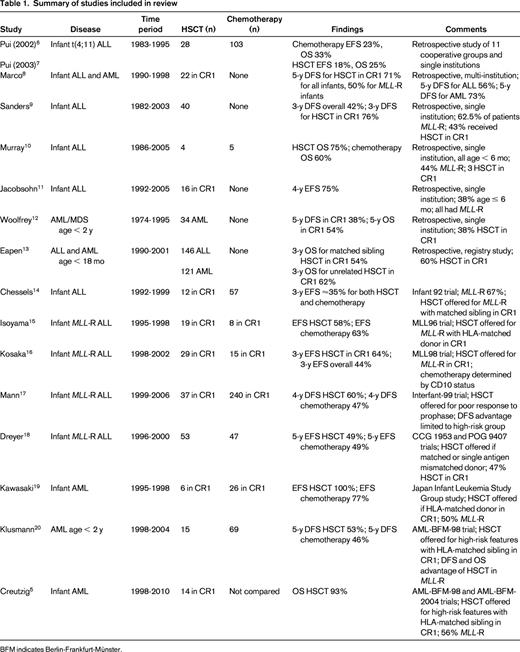
We performed a MEDLINE search for articles published between January 1998 and May 2013 to examine the evidence for the use of HSCT in infant leukemia. Using the keywords “leukemia,” “hematopoietic stem cell transplantation,” and “infant” yielded 529 results. After further limiting the search to those in the English language, we found 485 results. We reviewed each abstract and determined that 13 articles adequately addressed our question, from which we included an additional 3 articles from the bibliographies. In total, we included 16 articles (8 retrospective reports and 8 prospective studies) in our review (Table 1).
Pui et al performed a retrospective study of 497 children and young adults with MLL-rearranged (MLL-R) ALL who were treated by 11 cooperative groups and single institutions in the United States and Europe.6 After adjusting for initial WBC count, age, and time to transplantation, infants with t(4;11) who underwent any HSCT (n = 28) had a worse disease-free (DFS) and overall survival (OS) than those who received chemotherapy alone (n = 103).7 Further, matched sibling HSCT (n = 8) did not improve DFS or OS in infants with t(4;11) compared with chemotherapy alone. Marco et al reported 26 infants with acute leukemia (10 ALL, 15 AML, 1 bilineal) who underwent autologous or allogeneic HSCT at 7 Spanish hospitals.8 In the 22 infants who received transplantations in CR1, the 5-year DFS was 71% for all infants and 50% for those with MLL-R leukemia. Sanders et al reviewed a single-institution experience of 40 infants with ALL who underwent HSCT; 17 of the infants were in CR1 at the time of transplantation.9 The 3-year estimates of DFS were 76% in those who underwent HSCT in CR1 and 42.2% in the overall population. Eleven of 14 patients with MLL-R ALL who received transplantations in CR1 were in remission at the time of publication after a median follow-up of 7 years. Murray et al reported 9 infants diagnosed with ALL at a single institution.10 All infants were ≤6 months of age at diagnosis and 4 underwent allogeneic HSCT (3 in CR1, 1 after relapse). OS in their infants with ALL was 67% and 3 of the 4 patients who underwent HSCT survived (age at follow-up ranging from 18 to 66 months). Jacobsohn et al described 16 infants with ALL who underwent allogeneic HSCT in CR1.11 After a median follow-up of 4.7 years, 75% of the patients remained in remission. Woolfrey et al reported a single-institution experience of autologous and allogeneic HSCT in children under the age of 2 with AML (n = 34) and myelodysplastic syndrome (n = 6).12 Thirteen AML patients received transplantations in CR1; the 5-year DFS in these patients was 38% and OS was 54%. Eapen et al reviewed data from the Center for International Blood and Marrow Transplant Research and the New York Cord Blood Placental Program to compare survival in children 18 months or younger with ALL (n = 146) or AML (n = 121) receiving transplantations using a matched sibling donor versus an unrelated donor.13 Three-year leukemia-free and OS rates were not significantly different between the 2 groups. There were also no differences in recurrence, leukemia-free survival, or OS by type of leukemia. Chessels et al reported the results of the United Kingdom Medical Research Council Infant 92 study, which enrolled 86 infants with ALL.14 There were no differences in event-free survival (EFS) between those who underwent HSCT (n = 12) and those who received chemotherapy alone (n = 57). Comparing those with MLL-R who received HSCT and those who received chemotherapy alone also showed no differences in EFS (∼ 35% at 3 years for both treatments). Isoyama et al reported the results of the Japanese MLL96 study, which assigned 42 infants with MLL-R ALL to chemotherapy followed by HSCT in CR1 if an HLA-matched donor was available and 13 infants with non-MLL-R ALL to chemotherapy alone.15 Of the 42 MLL-R patients, 27 achieved a continuous complete remission after induction therapy, 19 received an HLA-matched HSCT, and 8 received continued chemotherapy or an autologous HSCT. After a median posttransplantation follow-up period of 615 days, 11 of the 19 MLL-R transplanted patients and 5 of the 8 MLL-R chemotherapy patients remained in complete remission. Kosaka et al reported the results of the subsequent Japanese MLL98 study that enrolled 54 infants with ALL with a specific goal of early HSCT (within 3-6 months of diagnosis) in infants with MLL rearrangements.16 Three-year EFS for all infants with MLL-R ALL was 43.6% and for those who underwent HSCT in CR1, it was 64.4%. Mann et al reported the outcome of patients receiving transplantations in the Interfant-99 study, an international trial that enrolled 297 infants with MLL-R ALL.17 After adjusting for time to transplantation, infants who underwent HSCT (n = 37) had a significant improvement in 4-year DFS and OS (60.1% and 65.6%, respectively) compared with those who received chemotherapy alone (n = 240, 46.8% and 48.6%, respectively). Subgroup analysis revealed that the improvements were limited to the high-risk group, defined by age < 6 months and either poor response to prednisone/prednisolone or elevated WBC count. Dreyer et al reported the results of parallel studies for infants with MLL-R ALL performed by Children's Cancer Group (CCG 1953) and the Pediatric Oncology Group (POG 9407).18 Fifty-three infants underwent HSCT and 47 infants received chemotherapy alone. The 5-year EFS rate was 48.8% in the HSCT group and 48.7% in the chemotherapy group, but the difference was not statistically significant. Kawasaki et al reviewed 35 infants with AML treated by the Japan Infant Leukemia Study Group.19 Thirty-two patients achieved CR1 after induction therapy and 6 patients underwent HSCT. All 6 patients remained in continuous complete remission at the time of the report compared with 20 of the 26 patients who received chemotherapy only. Klusmann et al reported the results of allogeneic HSCT in the AML-BFM-98 study, which included 247 children with high-risk AML.20 Five-year DFS in children under the age of 2 who underwent HSCT was 53% compared with 46% in those who received chemotherapy alone, but this difference was not statistically significant. Interestingly, when the overall study population was stratified by MLL status, those with MLL-R who underwent HSCT had a significant improvement in both DFS and OS (67% and 94%, respectively) compared with those who received chemotherapy alone (38% and 52%, respectively). Creutzig et al reported the combined results of 125 infants with AML treated on the AML-BFM-98 and AML-BFM-2004 studies.5 Fourteen infants with AML underwent HSCT in CR1 and 13 of the 14 patients survived. EFS and OS in high-risk infants with or without MLL-R was not significantly different and OS in high-risk infants was similar to that of older high-risk children.
Heterogeneity between the reviewed reports, including differences in chemotherapy and transplantation preparative regimens, time to HSCT, source of stem cells, sample sizes, patient characteristics, and the presence or absence of a chemotherapy comparison group, make it difficult to confirm definitively a benefit of HSCT in infant leukemia. In addition, none of the reviewed studies was randomized. As a result, confounding factors that may have potentially affected outcome were not equally distributed between the treatment groups because HSCT was only available to patients who met specific eligibility criteria (Table 1). Further, the sample sizes in each study were quite small, which also makes comparison between treatment groups more difficult. Finally, the Interfant-99 study suggested that high-risk patients were the only group who benefited from HSCT and future studies could use risk stratification based on age, response to therapy, and possibly the presence of minimal residual disease to identify patients who would benefit from HSCT.17,21 Based on our review, we conclude that there is insufficient evidence to support the use of HSCT in infant ALL or AML (grade 2C). Clinical trials specifically comparing HSCT with continued chemotherapy are needed to ultimately determine the role of HSCT in infant leukemia.
Disclosures
Conflict-of-interest disclosure: E.A.R.S. declares no competing financial interests. P.B. has consulted for Epizyme. Off-label drug use: None disclosed.
Correspondence
Edward Allan R. Sison, Division of Pediatric Oncology, the Bunting Blaustein Cancer Research Building, 1650 Orleans St, Rm 2M46, Baltimore, MD 21287; Phone: 410-502-7403; Fax: 410-955-8897; e-mail: esison1@jhmi.edu.