Abstract
Peripheral neuropathy (PN) is a frequent complication of plasma-cell dyscrasias such as monoclonal gammopathy of undetermined significance, multiple myeloma, Waldenström's disease, POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes) syndrome, Castleman's disease, and light-chain amyloidosis. PN can be associated with the underlying disease or it can related to the treatment. The novel immunomodulatory drugs thalidomide and lenalidomide and the proteasome inhibitor bortezomib have changed the standard treatment of multiple myeloma. Treatment-related PN induced by thalidomide (TiPN) or bortezomib (BiPN) has become the most frequent cause of symptomatic polyneuropathy in multiple myeloma and related diseases. Dealing with PN has become a major challenge in current clinical practice for multiple myeloma patients. This review deals with practical issues such as etiology, incidence, symptoms, and clinical management of treatment-emergent PN. The major focus of the hematologist should be on the prevention of PN, primarily by frequent monitoring of the patient and by timely and adequate dose reduction of thalidomide and bortezomib. Thalidomide should not be given for periods longer than 18 months, and if it is, then patients should be carefully monitored with a low threshold for discontinuation in the face of any emergent neuropathy. In the case of BiPN, the dose of bortezomib should be reduced and/or the administration interval should be prolonged from biweekly to weekly. Adequate pain management and supportive care require a multidisciplinary approach involving the treating physician, expert nursing staff, and a neurologist as clinically indicated.
Etiology of PN
Paraprotein-Associated PN
PN is defined as damage to or degeneration of peripheral nerves involving sensory, motor, or autonomic nerve fibers. Using clinical and electrophysiological assessments, baseline PN may be observed in up to 54% of newly diagnosed patients with plasma cell dyscrasias, including monoclonal gammopathy of undetermined significance (MGUS), multiple myeloma, POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes) syndrome, Waldenström's macroglobulinemia, light-chain amyloidosis, and Castleman's disease.1–3
PN can be secondary to the monoclonal protein or it can be the result of compression of the nerve roots. Although the exact etiology is not known, IgM antibodies binding to myelin-associated glycoprotein in nerves may target neural antigens of the myelin sheet, thereby damaging interactions between Schwann cells and axons.4 The relationship of the IgG or IgA monoclonal gammopathy with polyneuropathy is less clear (M. Eurelings, unpublished data). Small-fiber neuropathy is often seen in amyloidosis, and may be present as a result of pro-inflammatory cytokines and vasoactive peptides.1–3,5 Cryoglobulins are associated with nerve damage and amyloid deposits are found in patients with light-chain amyloidosis and PN.
PN Induced by Bortezomib and Other Proteasome Inhibitors
Bortezomib, a reversible inhibitor of the 26S proteasome subunit β5, and carfilzomib (NPI-0052), an irreversible inhibitor, prevent proteasomal degradation of ubiquitinated proteins.6 Bortezomib causes a preferential sensory neuropathy.5,7,8 In small animals, the accumulation of ubiquitinated proteins in dorsal root ganglia has been observed, and aggregates seen in the cytoplasm of neuronal and supportive cells suggest a specific class effect of proteasome inhibitors.9 The combination of bortezomib with Ca2+ modulators in vitro induces mitochondrial-mediated apoptosis, indicating a critical step in bortezomib cytotoxicity in neurons.10 Other potential mechanisms of bortezomib-induced PN (BiPN) include blockade of the transcription of nerve growth factor through nuclear factor κB inhibition, increased tubulin polymerization, and microtubule stabilization.11 The observation that bortezomib causes reactivation of Varicellazoster underlines the potential role of proteasome inhibition in the dorsal ganglia.12
In addition to toxicity to the dorsal ganglia, detrimental effects to the axons downstream extend into the peripheral nervous system and in particular to the small fibers, thus contributing to the development of PN, especially in the most vulnerable, longest axons. In contrast to the boron-based bortezomib, the epoxyketone-based and irreversible proteasome inhibitor carfilzomib does not appear to damage the dorsal ganglia and downstream axons to the same degree, although the effects on the dorsal root ganglia do appear to be a class effect of all proteasome inhibitors studied in preclinical models to date. Commensurate with this, phase I and II trials of carfilzomib have demonstrated substantially less clinical neurotoxicity than that seen with the boronates, with treatment-emergent rates of PN estimated at up to 15%, compared with approximately 30% with bortezomib.13
Immunomodulatory Drug-Induced PN
Thalidomide, lenalidomide, and pomalidomide belong to the class of immunomodulatory drugs. Thalidomide effects include inhibition of angiogenesis, immunomodulation, anti-proliferative activity, and induction of apoptosis. While the mechanism of thalidomide-induced PN (TiPN) is not clear, inhibition of vascular supply to nerves, direct toxic effects on dorsal ganglia, and down-regulation of tumor necrosis factor-alpha (TNF-α) leading to demyelinization have been proposed.7,14 In contrast to BiPN, thalidomide results in a mixed sensory-motor type of neuropathy caused by preferential degeneration of the most vulnerable, longest axons, which may be irreversible with continued use.7,15,16
PN Caused by Chemotherapy
A variety of cytotoxic drugs are associated with neuropathy, including vinca-alkaloids, taxanes, and platinum compounds.17 Vincristine binds to tubulin and inhibits microtubule stabilization, causing distal axonal degenerative neuropathy. Cisplatinum causes direct damage to the dorsal ganglia and sensory neurons. Other neurotoxic cytostatic drugs such as taxanes are rarely used in multiple myeloma and will not be discussed herein.
Clinical Presentation of PN
Clinical Characteristics and Symptoms
Baseline PN is sensory or sensorimotor as result of axonal degeneration, usually presenting as symmetrical paresthesias, numbness, weakness, or burning pain.2 PN in multiple myeloma patients may differ from IgM-associated PN based on the demyelinating mechanism, resulting in weakness, foot drop, and abnormal gait.1
TiPN is predominantly sensory and sensorimotor.14 It usually starts within months, but may present even after thalidomide has been discontinued.19 Sensory PN usually presents as symmetric hypesthesia (numbness), tingling, or hyperesthesia of fingers and toes. Because the longest nerves may be the first affected, symptoms typically start in the distal end of the extremities and may progress to proximal nerves.15 Motor PN occurs less frequently and primarily in patients with severe sensory PN19 ; it may present as muscle cramps, tremor, or weakness. Reduced distal muscular weakness such as foot drop should be discriminated from steroid-induced myopathy, which affects proximal muscles, resulting in difficulty rising from a chair or climbing stairs. Autonomic TiPN is associated with orthostatic hypotension, bradycardia, constipation, and sexual dysfunction, and may be caused by small-fiber damage.20 PN by lenalidomide and pomalidomide is less frequent and less severe, because these agents have mostly been used in combination with corticosteroids21,22 (Table 1).
BiPN is typically sensory, characterized by hyperesthesia, hypoesthesia, paresthesias (tingling), and altered temperature sensations in the hands and feet.5,23 Burning sensations or pain may occur at rest, and are usually localized in the soles of the feet, but occasionally also present in the fingers and the palmar sides of the hands. These sensations are caused by damage to small fibers, which in BiPN is in the form of reduced intra-epidermal nerve fiber density.24 Symptoms usually start in the distal extremities and progress proximally.5,24 Although BiPN most frequently is mild, severe and rapidly progressing cases have been reported. Motor PN from bortezomib is less frequent and follows sensory PN. Autonomic PN is also observed following bortezomib treatment. Second- and later-generation proteasome inhibitors less frequently induce PN, although available data are still limited.
In clinical practice, PN is graded using the Common Toxicity Criteria of the National Cancer Institute (NCI-CTC version 3.0; Table 2).
Incidence of PN
The reported incidence of treatment-induced PN is influenced by the accuracy of PN assessment, patient selection, dosing, schedule and duration of treatment, co-medications, and comorbidities.
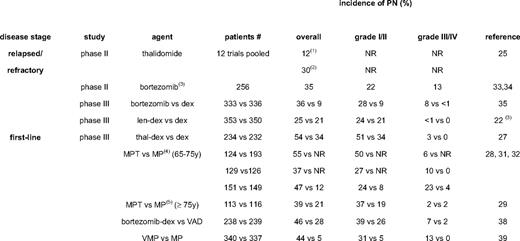
TiPN was reported as early as 1961, and is the most frequent toxicity of thalidomide, with an incidence ranging from 25% to 75% (Table 3). Unfortunately, dosing of thalidomide throughout clinical trials has been extremely variable, which has contributed to a higher incidence of severe PN. A systematic review of thalidomide monotherapy in relapsed multiple myeloma reported an overall PN incidence of 12% to 44%; a grade 1 and 2 PN incidence of 22%, and a grade 3 and 4 PN incidence of 6% (Table 3).25 Thalidomide combined with dexamethasone in relapsed/refractory multiple myeloma is associated with a slightly lower overall PN incidence of 27%.25,26
TiPN is increasingly used in the frontline treatment of multiple myeloma in elderly patients (melphalan/prednisone/thalidomide, MPT; thalidomide/dexamethasone, TD) or in induction regimens for transplantation candidates (TD; thalidomide/adriamycin/dexamethasone, TAD). The reported incidence of grades 2 and 4 TiPN are up to 50% with MPT and 31% with TAD.27–33 Awareness of PN has now reduced its incidence through the avoidance of prolonged administration and by timely dose reduction, resulting in a less than 10% incidence of grades 3 and 4 PN. Motor PN occurs less frequently, but may be complicated by steroid-induced myopathy. Thalidomide-induced constipation is a frequent symptom of TiPN and occurs in as many as 75% of patients. Large clinical trials with lenalidomide in relapsed/refractory multiple myeloma have shown a significant reduced overall incidence of PN compared with thalidomide, and no grades of 3 or above.22 Data on PN with pomalidomide have not yet been published, but meeting abstracts do not report PN grades of 3 or above.
BiPN has been well documented from the time of its initial clinical introduction. Dosing of bortezomib has been strict, using an algorithm of dose adaptation in cases of treatment-emergent PN. In phase II and III trials (SUMMIT, CREST, and APEX in relapsed/refractory multiple myeloma), BiPN was observed in 37% (all PN), 22% (grades 1 and 2 PN), 13% (grade 3 PN), and 1% (grade 4 PN), respectively (Table 3).34–36 Interestingly, BiPN was less frequent with a lower dose or weekly schedule of bortezomib.35,37 The use of bortezomib in frontline regimens prior to transplantation was associated with BiPN in 47% of patients, with up to 16% being grade 3 or 4.38 In a phase II trial followed by the VISTA randomized phase III trial, in elderly patients bortezomib/melphalan/prednisone (VMP) was associated with 13% grade 3 and 44% overall BiPN, with a median bortezomib dose to onset of PN of 32 mg/m2 and a maximum cumulative incidence after 45 mg/m2.39–41 Sensory PN was the main reason for reducing (22%) or stopping (11%) bortezomib. The incidence of BiPN in recent trials is shown in Table 3. Retreatment with bortezomib does not seem to increase PN. Interestingly, the rate of BiPN is lower when bortezomib is administered combined with thalidomide/dexamethasone (VTD) or lenalidomide/dexamethasone (VRD), resulting in 2% to 3% grade 3 PN in frontline and relapse treatment.42,43 This may be explained by the anti-inflammatory effects of the immunomodulatory drugs and/or dexamethasone or more prompt dose reductions because of increased awareness. Carfilzomib seems substantially less neurotoxic, while NPI 0052 is still under clinical testing and the doses tested have not yet been shown to be therapeutic.
Risk Factors for PN
Baseline PN is present in 54% of patients with myeloma. Therefore, it is of vital importance to identify predisposing factors for treatment-emergent PN, such as comorbidities and other variables. The presence of diabetes mellitus, alcohol abuse, vitamin deficiencies, and viral infections represent a baseline condition that may elicit or aggravate symptomatic PN. Age by itself does not seem to be a predisposing factor. In the relapsed/refractory setting, prior treatment with neurotoxic agents such as vincristine, thalidomide, or bortezomib did not affect the risk of grades 3 and 4 BiPN. In the VISTA trial, a history of neuropathy was the only consistently strong risk factor for any PN, for grade 2 PN and above, and for grade 3 PN and above.40
Recently, pharmacogenetic studies have been performed in newly diagnosed patients who were treated with thalidomide or bortezomib in prospective clinical trials. It has been postulated that pro-inflammatory proteins and vascular mediators may contribute to the emergence of PN, and this is reflected in primary tumor cell gene-expression profiles. A recent pharmacogenomic analysis from the phase III HOVON65/GMMG-HD4 and IFM 2005–1 trials, which compared VAD (vincristine/doxorubicin/dexamethasone) with PAD (bortezomib/doxorubicin/dexamethasone) and VD (bortezomib/dexamethasone), respectively, found that single nucleotide polymorphisms in genes involved in inflammation (such as the proinflammatory cytokines TNF-α, PARP1, and MBL2) and neurological processes (such as IKBKAP, SERPINB2, and DPYD) are significantly associated with the development of BiPN.44 Significant associations were also seen with ADME (absorption, distribution, metabolism, and excretion) genes such as the ABC and CYP genes. Similarly, an independent pharmacogenomic analysis of the VISTA trial identified an association between time to onset of PN and the immune gene CTLA4, and the same association was shown in a confirmatory analysis of data from the IFM trial mentioned above.45 Efforts are under way to investigate whether single nucleotide polymorphisms can be used to identify patients at risk of developing BiPN.
An analysis of the genetic factors that affect TiPN also identified significant associations with single nucleotide polymorphisms in the ABC genes (ABCC1) and in neurologic genes such as SERPINB2, indicating that TiPN may also be associated with neuroinflammation and/or cumulative damage or the inability to repair neuronal damage, while the identified genes were distinct from those associated with BiPN. Little overlap was observed with the genetic factors that affect survival or with vincristine-induced PN.46
Reversibility and Outcome of PN
For the management of treatment-emergent PN, it is important to make a priori choices based on expected reversibility and long-term outcome. Limited damage to the peripheral nerves may resolve, while more severely damaged neurons or dorsal ganglia may take longer to regenerate.
Thalidomide causes permanent nerve damage, and consequently the clinical reversibility of TiPN is moderate or none. The dosing and duration of treatment seems of critical importance. Time to onset of TiPN is within months, and may be even faster with higher doses. TiPN was consistently higher in patients treated with daily doses greater than 200 mg compared with doses of 50 to 200 mg.25 Some authors suggest that cumulative doses that exceed 20 to 40 g are correlated with higher PN frequencies. After thalidomide withdrawal, a temporary flare-up of paresthesias has been observed. However, after stopping thalidomide, up to 90% of patients with a TiPN grade of 2 or above were shown to improve to a lower grade of TiPN within 4 months, as shown by electrophysiological monitoring, while others took years to recover.47 These observations have prompted the recommendation for immediate dose reduction or discontinuation as soon as signs of TiPN develop.18,48,49 Limiting the duration of thalidomide exposure to 6 to 12 months maximum is also recommended.
Bortezomib causes mostly reversible PN. BiPN occurs after a median of 3 months, with a plateau reached after 5 to 6 cycles. In a subset of patients, painful BiPN can emerge subacutely during an early phase of treatment (even during the first cycle). Algorithms for dose reduction and holding have been proven effective, and these should be accurately applied in a timely manner.39 Clinical BiPN resolves in 64% of affected patients with grade 2 to 4 within 3 to 4 months. Improvement usually takes longer with grades 3 and 4 PN than with minor symptoms. Electrophysiological studies in animals have confirmed functional recovery.37,39 Because of BiPN reversibility, retreatment with bortezomib is feasible and no cumulative BiPN has been observed.50
Diagnosis of PN
Early detection of treatment-related PN is required for timely and appropriate intervention. Therefore, procedures in daily clinical practice should implement uniform and practical tools for screening and grading of PN. NCI-CTC criteria, or more specific scales such as the Functional Assessment of Cancer Therapy Neurotoxicity (Ntx-FACT/GOG) subscale (Table 2), the reduced Total Neuropathy Score (TNSr),51 CI-PERINOMS,52 and the EORTC CIPN2053 can be used, although these do not all evaluate neuropathic pain such as that observed with BiPN. In addition to these patient-focused tools, symptom registration and a regular and focused clinical neurological examination including the evaluation of sensation (touch, pain, temperature, vibration, proprioception), distal muscle strength, ankle reflexes, and supine versus upright blood pressure is recommended. More complex algorithms such as the TNSr combine the assessment of symptoms with electrophysiological measurements, resulting in a higher sensitivity for the detection and specification of neurological damage. Nerve-conduction studies and needle electromyography can help to quantify the severity of neuropathy and to differentiate treatment-related (mostly primarily axonal neuropathies) from disease-related (often demyelinating) PN and from non-neurological problems.
TiPN is associated with axonal sensorimotor polyneuropathy with reductions in sensory nerve action potentials and distal compound motor action potentials. On needle examination, denervation and re-innervation in distal muscles can be observed. BiPN is also associated with axonopathy, with a concomitant decrease in nerve-conduction velocity due to demyelination. Unfortunately, neurophysiological and clinical symptoms may vary in TiPN and BiPN. Abnormal baseline nerve-conduction studies do not reliably predict developing TiPN, because patients with limited PN more frequently have involvement of larger axons. In contrast, small-fiber damage, as frequently occurs in BiPN, will result in fewer abnormalities, as shown in the nerve-conduction studies.
Management of PN
Prevention and Prophylaxis
Prevention of PN is the preferred approach to maintaining the daily quality of life and the level of activities and to preserve future options for anti-myeloma treatment. All patients should be evaluated for clinical signs and symptoms of PN before the administration of a neurotoxic drug. Patients with preexisting PN should be followed even more closely, because they are at risk for more severe PN. Patients who receive neurotoxic treatment should be monitored at each visit with standard examination and with a neurologic examination in case of suspected PN. Typically, this requires a multidisciplinary approach involving trained nurses, hematologist-oncologists, and neurologists.
Dose and Schedule
In order to prevent and reduce PN, dosing algorithms have been developed for thalidomide and bortezomib (Table 4). Although guidelines for the reduction of thalidomide have not been validated and are largely based on expert opinion, they offer a practical tool for drug dosing in case of developing PN. Limiting the initial thalidomide dosing to 200 mg daily is recommended to prevent the rapid development of TiPN. The dose of thalidomide may be reduced to 50 to 100 mg for maintenance treatment. Dose reduction to 50 to 100 mg is mandatory once PN grade 1 occurs, and thalidomide must be discontinued completely in cases of grade 3 PN and above. Because TiPN is correlated with the total cumulative dose, the drug should not be given for longer than 6 to 12 months.
The guidelines for bortezomib were initially based on experience developed in the single-agent CREST and SUMMIT trials, and are evidence-based dose-modification tools that were subsequently validated in the APEX and VISTA trials. The recommendations indicate when bortezomib dosing should be reduced or discontinued (Table 4). Frequent monitoring and adequate use of the guidelines have been demonstrated to result in a reduction of severe PN without affecting clinical efficacy.37,39 More recently, multi-agent studies have shown that, in addition to dose reduction, weekly dosing may prevent the progression of PN and reduce severe PN (≥ grade 3).54 Weekly bortezomib dosing is therefore considered to be an effective strategy to prevent further worsening of PN once patients have developed PN grade 1. Based on expert advice, this approach is currently recommended, and will be implemented in the International Myeloma Working Group (IMWG) consensus guidelines. Once PN has resolved, there is no increased risk for cumulative BiPN upon retreatment with bortezomib-containing regimens. The dose recommendations for thalidomide and bortezomib are summarized in Table 4.
Pharmacological Intervention and Pain Management
In spite of the clinical importance and high incidence of treatment-emergent PN, few successful intervention strategies for prevention have been developed. There are no prospective randomized studies evaluating prophylactic pharmacologic interventions. A wide variety of supplements have been used, including vitamin B, antioxidants such as vitamin E, alpha-lipoic acid, glutathione, and glutamine or acetyl-L-carnitine. In a prospective evaluation by Richardson et al., some potential beneficial effects from the use of supplements was observed, with lower rates of grade 3 BiPN being found.24 Vitamin B12 and folic acid deficiencies should be corrected. High-dose vitamin C is not recommended because it interferes with bortezomib. Alpha-lipoic acid has been approved in Europe for the treatment of diabetic neuropathy, and is effective in docetaxel-induced PN55 ; however, in animal models, there are indications that it may reduce bortezomib efficacy. The evidence of supplements in multiple myeloma remains anecdotal, and is largely based on extrapolation from trials with other neurotoxic drugs in different diseases.56 Prospective studies have been limited, and there are no results from controlled clinical trials to date. While their usefulness for BiPN or TiPN is not proven, reports of benefit suggest that supplements may have a complementary role.57 However, they should not be used on the same day as bortezomib administration and they should certainly not be used as an alternative to the dose-reduction guidelines. There is a need for further prospective, randomized trials to better define the role of supplements for PN.
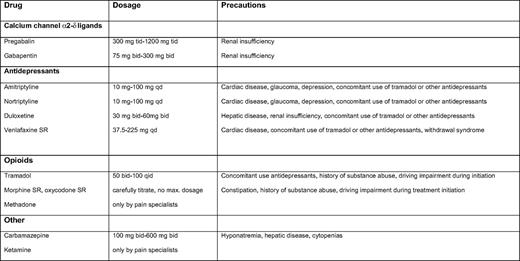
The recommended first-line treatment for neuropathic pain in multiple myeloma includes calcium channel α2-δ ligands (i.e., gabapentin and pregabalin) and certain antidepressants (i.e., tricyclic antidepressants and dual reuptake inhibitors of both serotonin and norepinephrine); Table 5 contains information on dosing and schedule. Opioid analgesics and tramadol are recommended as second-line treatments that also can be considered for first-line use in selected clinical conditions such as acute and severe pain. Other medications that are generally used as third-line treatments include anti-epileptics and ketamine, but these could also be used as second-line treatments in selected patients (Table 5).58 The advice to use these agents is based on case reports, personal experience, or on studies of other indications such as painful diabetic neuropathy and post-herpetic neuralgia, not on studies in TiPN or BiPN. Local application of analgesic cream to painful skin areas has only been proven to be effective for small, localized areas of PN. Emollient creams such as cocoa butter and menthol-based preparations can be used to alleviate local complaints.57,59 Patients may be advised to wear loose-fitting clothes and shoes and to keep feet uncovered in bed.
In addition, patients with (severe) PN experience difficulties with daily tasks, and may benefit from physical exercise and physiotherapy. Patients with autonomic dysfunction should rise cautiously and avoid demanding physical tasks. Hydration (500 cc saline) during bortezomib administration may reduce the incidence and severity of dizziness and fainting.
Conclusions
PN is present in up to 50% of patients with plasma-cell dyscrasia at diagnosis, although the incidence of clinically significant PN is lower. PN is also a frequent and dose-limiting side effect of most current anti-myeloma treatments, in particular thalidomide and bortezomib. For many patients, the probability of prolonged remission depends on the treatment with these agents combined or during successive stages of their disease. It is therefore imperative to prevent TiPN and BiPN in order to allow the treatment to be continued and completed. The prevention of severe PN by close monitoring, patient reporting by questionnaires, and most importantly by using dose-reduction algorithms defines the standard of care. Hopefully, pharmacogenomic and predisposing factors can be identified in future studies to provide better recognition of high-risk patients. Prospective trials of pharmacologic interventions are also needed.
Acknowledgments
The authors wish to thank our colleagues from the International Myeloma Working Group (IMWG), and specifically the Peripheral Neuropathy team (M. Beksac, M. Delforge, C. Mitsiades, P. Richardson, P. Wen), as well as the European Myeloma Network (EMN) for continuing discussions and assistance.
Disclosure
Conflict-of-interest disclosure: The authors declare no competing financial interests. Off-label drug use: None disclosed.
Correspondence
Prof. P. Sonneveld, MD, Erasmus MC, Dept of Hematology, Rm L 407, P.O. Box 2040, 3000 CA Rotterdam, the Netherlands; Phone: 31–10-7033123; Fax: 31–10-7035814, e-mail: p.sonneveld@erasmusmc.nl