Abstract
Allogeneic hematopoietic stem cell transplantation (allo-SCT) remains the only proven cure for chronic myeloid leukemia (CML), a rare malignancy in childhood. With the excellent results induced by the tyrosine kinase inhibitor (TKI) imatinib in adults in the last decade, the appropriate management of children with CML has also changed radically, and only a minority are now transplanted as a front-line treatment. Data on pediatric experiences with imatinib in CML from controlled trials remain very limited, but this review of available data describes the role of imatinib in children with CML, addressing: 1) the starting dose; 2) pharmacokinetics in childhood; 3) possible adverse effects, with a focus on the still-growing skeleton; 4) early monitoring of treatment efficacy in an attempt to avoid failure; 5) the timing of allo-SCT in children; and 6) treatment of CML relapse after allo-SCT. Because the characteristics of CML in children seem to overlap extensively with what is described in adult internal medicine, most answers and pediatric algorithms are adapted from the treatment of CML in adults. Today in 2010, allo-SCT in children should be postponed until CML becomes refractory to imatinib. The approach for young patients with suboptimal responses is unclear because data on the efficacy and safety of second-generation TKIs in childhood are almost entirely missing. Other than being included in a formal trial on second-generation TKIs, allo-SCT for patients failing imatinib remains the first choice.
Introduction
Chronic myeloid leukemia (CML) in childhood is rare, accounting for less than 10% of all cases of CML and less than 3% of all pediatric leukemias. Incidence increases with age, being exceptionally rare in infancy at 0.7/million/year at ages 1 to 14 years and rising to 1.2/million/year in adolescents.1 As a consequence of its rarity, only a few studies have specifically addressed pediatric features of CML at diagnosis and the treatment outcome. Generally, children are diagnosed at a median age of 11 to 12 years (range 1–18 years), with approximately 10% presenting in advanced phases. Leading symptoms are asthenia (45%–60%), splenic discomfort (20%–30%), weight loss (15%–20%), and bleeding (10%). The median white blood cell count (WBC) is 240 × 109/L (range, 10–720 × 109/L), which is higher than in the adult population; anemia or thrombocytosis are also present in 60% of the children.2 Splenomegaly (60%–70%) is more common in patients with higher WBC, in whom the platelet count tends to be lower.3 The biology of CML has been extensively reviewed.4 In both children and adults, the natural course of the disease is progression from the chronic phase (CML-CP) to the advanced phase (CML-AP) and then the blastic phase (CML-BP), but treatment responses generally do not seem to differ. CML-BP is generally either myeloid (60%–80%) or lymphoid (20%–30%), but a mixed-lineage phenotype also has been observed. Rarely, patients may present in CML-BP without a diagnosed preceding CML-CP. In this situation, discrimination between CML-BP and Philadelphia chromosome-positive (Ph+) acute leukemia may be impossible.5
The molecular pathogenesis of CML involves the BCR-ABL (breakpoint cluster region-Abelson leukemia virus) fusion proteins, whose constitutively increased tyrosine kinase activity contributed by the ABL component appears to be the causative molecular alteration in CML. Identification of this association resulted in the successful development of imatinib mesylate (trade names Gleevec® or Glivec®, Novartis Pharmaceuticals). Imatinib is a potent inhibitor of the ABL tyrosine kinase, and also inhibits other subclass III receptor tyrosine kinases, including c-kit and PDGF-R (platelet-derived growth factor receptor).6,7 The concept of tyrosine kinase inhibition has been examined in several studies, and has within a decade dramatically improved the perspective for adult patients with CML.6 A recent 7-year update of the phase III IRIS (International Randomized Study of Interferon versus STI571) trial confirmed the long-term efficacy and safety of imatinib, and the drug is currently recommended as the first-line therapy for CML-CP by both the National Comprehensive Cancer Network (NCCN) and the European Leukemia Net (ELN).8–10 As a consequence, the role of allogeneic hematopoietic stem-cell transplantation (allo-SCT) in first remission is becoming less clear. While the results of allo-SCT for CML are best in pediatric patients, there are no data with respect to long-term use, and therefore the safety of tyrosine kinase inhibitors (TKIs) in children.11 This review seeks to provide some guidance as to the appropriate management of CML in childhood.
Treatment without TKIs
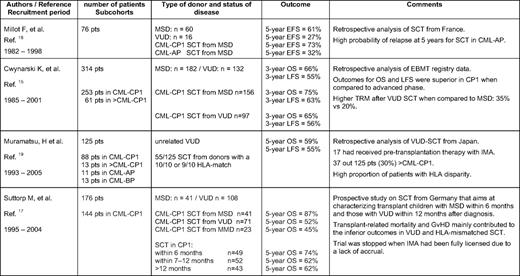
Prior to the availability of imatinib, the initial treatment with hydroxyurea, followed by interferon-alpha (IFN-α) with or without cytosine arabinoside, was the routine clinical management for children with CML before undergoing allo-SCT, a procedure recommended for all patients with a matched donor. Published pediatric results on IFN for CML are scant. Overall, no significant differences between children and younger adults with respect to response rates (complete hematological response [CHR], 58%; major cytogenetic response [MCyR], 50%; complete cytogenetic response [CCyR], 14%) or overall survival (OS; 60% at 8 years) have been observed in small cohorts.12,13 While prognostic scores based on clinical and biological features at diagnosis (i.e., the Sokal, Hasford, and Kantarjian Scores) have proven their usefulness in predicting the time to progression of CML with a defined treatment applied in adults, a pediatric CML scoring system has not yet been established. This limitation is basically due to the fact that in the past 20 years, most children with a matched donor underwent transplantation soon after diagnosis and the side effects of allo-SCT imposed a far greater impact on survival than the natural course of CML. Results of allo-SCT in CML from studies in adults are age-dependently superior in younger patients. Table 1 gives an overview of the results from the larger studies of pediatric patients (N > 50) published in the last 10 years. Generally, OS ranges between 60% and 80%, with better results in matched sibling donors compared with matched voluntary unrelated donors.14–20 Interestingly, while the German prospective trial was stopped because of lack of accrual after imatinib had been licensed, the most recent report from a Japanese group already included 13% of patients with prior imatinib treatment.17,19 With the exception of the German study, no pediatric studies have evaluated the optimal conditioning regimen for childhood CML. In agreement with data from adults comparing total body irradiation with busulfan/cyclophosphamide regimens, no significant differences on OS either in the matched sibling donor or the matched voluntary unrelated donor setting were detected.17 However, because the long-term sequelae of total body irradiation have to be considered, most pediatric allo-SCT physicians prefer busulfan/cyclophosphamide as the conditioning regimen for CML-CP.
Treatment with TKIs
Although imatinib has dramatically reduced the yearly risk of CML progression, it does not cure the disease, and in most patients leukemic stem cells will persist. Nevertheless, imatinib must currently be considered to be the best front-line treatment for CML, a benefit that should be offered to children11,21–24 The drug was licensed for the use in children by the Food and Drug Administration in 2003. To date, the efficacy and side effects of imatinib have been evaluated in controlled phase I/II trials comprising less than 200 children with Ph+ leukemias and solid tumors.22–30 Doses of 260 to 340 mg/m2 give drug exposures similar to the 400 to 600 mg adult dosage levels; therefore, the starting dose in children should be 300 mg/m2 orally once daily (maximum absolute dose, 400 mg). The recommended pediatric doses for CML-AP are 400 mg/m2 daily (maximum absolute dose, 600 mg) and for CML-BP 500 mg/m2 daily (maximum absolute dose, 800 mg). Female teenagers must be advised to avoid conception while taking imatinib, because the rate of fetal abnormalities was shown to be higher than expected in a comparable healthy population.31
TKI treatment response is measured based on hematologic, cytogenetic, and molecular tests. By making use of blood counts and differentials, the hematological response can be assessed, while the examination of marrow cell metaphases evaluates the CyR, and a quantitative reverse-transcriptase polymerase chain reaction (RT-PCR) allows the assessment of BCR-ABL transcripts level, which shows the molecular response (MolR). Recently, the ELN (http://www.leukemia-net.org) has proposed recommendations for the medical management of CML patients of all ages.10 Close monitoring of treatment response, short- and long-term side effects, and prognostic factors is required. In particular, the development of imatinib resistance, intolerance, noncompliance, or progression to advanced-phase disease must be identified in a timely fashion. In addition, the treatment benefits of second-line therapies (i.e., allo-SCT, second-generation TKI) must be considered. This basically means that imatinib should be continued in cases of optimal response, while in cases of failure to respond, second-generation TKIs and/or allo-SCT should be considered. In cases of suboptimal response, treatment with imatinib may be continued at the same or higher dosage, but some patients may become eligible for second-generation TKIs. As long as no specific recommendations for children can be made, it might be advisable to follow the guidelines for monitoring minimal residual disease (MRD) in adults.8,10 Whenever possible, pediatric patients with CML should be enrolled in clinical trials and treated under the guidance of an experienced pediatrician affiliated with a center that can facilitate appropriate cytogenetic and molecular monitoring. It must be stressed that the sensitivity level for the detection of low numbers of BCR-ABL transcripts varies for different laboratories. Significant advances toward the standardization of quantitative RT-PCR for BCR-ABL have been made, with a proposal for an international scale and the development of procedures for testing laboratories to derive conversion factors to that scale.32 The ongoing development of reference reagents should enable more laboratories to use the international scale and thus facilitate the introduction of better internal quality assurance systems.
Pharmacokinetics
Data on imatinib pharmacokinetics are reported for a limited number of children receiving the drug in doses of 260 to 570 mg/m2/d.22,27–29 Despite a wider inter-patient variability in children, the plasma drug levels were similar to those reported in adults treated with standard doses of 400 to 600 mg/d. However, the main circulating metabolite of imatinib, N-desmethyl-imatinib (CGP 74588), which is as active as imatinib, was eliminated at steady state with a half-life of 16 h, similar to the parent drug but shorter than that observed in adults. Most probably the metabolism of CGP74588 is more dependent on plasma concentration and interactions than that of imatinib. The clearance of imatinib was apparently positively correlated with body weight and albuminemia and negatively correlated with plasma alpha-1-acid glycoprotein.29 The only way to perfectly control the imatinib concentration is to apply therapeutic drug monitoring, and this has been proposed for the management of CML. Indeed, it was shown in adults that mean trough plasma concentrations of imatinib were significantly lower in patients without a CCyR.30
Side Effects of Imatinib
Imatinib toxicity is common but the effects are generally mild to moderate.33,34 So far, it can be deduced from small cohorts that side effects occur with the same or even a lower frequency and are less severe than in adults.23,26,28 Toxicity includes nausea, vomiting, diarrhea, skin rash, edema, elevated liver enzymes (mild transaminitis generally occurs early in the course of treatment), and cytopenias. Especially in the beginning of therapy or when advanced stages of CML are treated, the onset of cytopenias may require short treatment interruptions because of an inadequate reservoir of normal stem cells. Treatment of neutropenia with granulocyte colony-stimulating factor (G-CSF) in some patients is also an option. The absorption and metabolism of imatinib may be affected by other concomitant medications, and possible adverse effects of drug interactions must always first be excluded. Children mostly complain about nausea. Because imatinib absorption is not affected significantly by administration with food, taking imatinib with meals and generous fluids (e.g., apple juice) is recommended in order to minimize upper gastrointestinal tract toxicity. Some patients may experience lethargy and some gain weight. Myalgia/cramps (usually mild to moderate and located in the hands, feet, calves, and thighs) are also very commonly observed; these are variable in pattern, frequency, and severity, and occur more frequently at night or with exertion. The management of bone pain, which may occur in up to 10% of children and tends to fade with longer therapy, should generally be managed supportively with anti-inflammatory agents and analgesics as needed. Much attention has been given to the potential cardiotoxicity of imatinib, the role of which is to inhibit ABL in cardiac tissue, which was shown to cause detrimental effects on the viability of cardiomyocytes in animal models.35,36 The consensus interpretation of these data from large cohorts in adult patients is that cardiac toxicity is very rare with imatinib, and not a concern that should influence clinical decision making. However, this might be a worrisome issue for long-term therapy in children.
Bone Metabolism and Longitudinal Growth
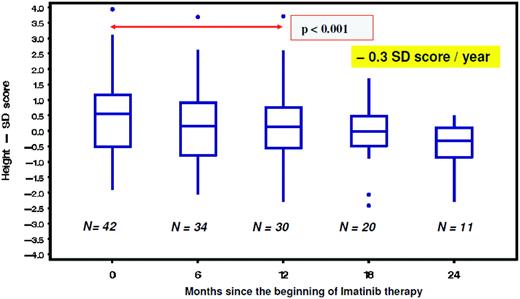
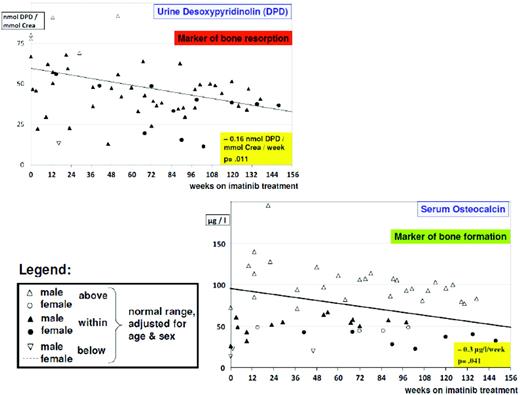
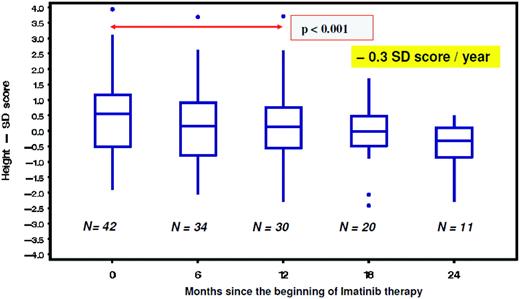
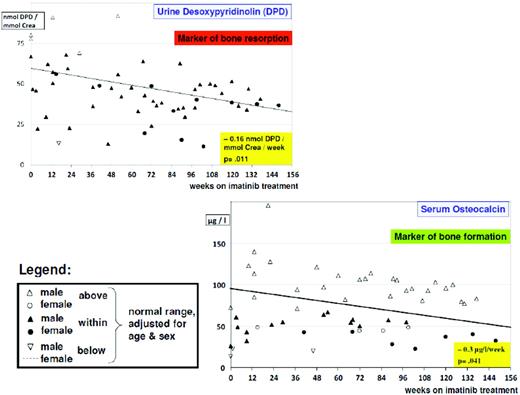
From studies in adults, imatinib is well known to cause hypocalcemia and hypophosphatemia. There is increasing evidence suggesting that dysregulated bone remodeling may be a side effect of TKI treatment. This likely results from inhibition of osteoclasts by blocking PDGF-R, c-fms, c-kit signaling, reductions in the activity of human carbonic anhydrases II and XIV, and increases in osteoblast activity through the inhibition of PDGF-R.37 While this has led to the suggestion that imatinib may increase bone strength through positive effects on bone mass density and trabecular volume in adults, it was shown in the not-outgrown skeleton of juvenile animals that the femoral length of mice is reduced dose dependently by chronic exposure to imatinib.36 Moreover, premature growth plate closure was reported in rats.38 In prepubertal children, three single case reports have demonstrated massive growth retardation.39–41 An ongoing French pediatric CML phase IV trial reported retrospectively collected data on 30 children exhibiting a highly significant (p < 0.0001) decrease of body height standard deviation scores (SDS) with a median of the difference of –0.37 SDS (range, –1.09–0.14 SDS) between the start of imatinib treatment and 12 months later (Figure 1).42 In a German trial on 57 children treated with imatinib, a prospective analysis of bone biochemical parameters demonstrated hyperparathyroidism in 25% of the cohort. Increased levels of the bone resorption marker CTX I (C-terminal cross-linking telopeptide of collagen type I) were seen over the entire treatment period in 60% of the patients, while a significant decline in desoxypyridinolin (DPD) was observed during ongoing therapy (Figure 2). Osteocalcin levels as marker of bone formation were elevated at the beginning of imatinib therapy and declined significantly during further treatment before reaching and holding normal range.43,44 Taken together, these data clearly show that children, especially if prepubertal, are at an increased risk of impaired bone remodeling if treated with TKI, which may result in growth retardation. Careful monitoring of longitudinal height as well as serum calcium, phosphate, parathyroid hormone, and bone metabolic markers seems to be mandatory. However, the optimal approach for treating children suffering from this side effect remains undefined.
Decline of body height as indicated by a significant (p = 0.001) decrease of the standard deviation (SD) score in children with CML receiving imatinib treatment.42
Decline of body height as indicated by a significant (p = 0.001) decrease of the standard deviation (SD) score in children with CML receiving imatinib treatment.42
Alteration of bone biochemical markers in children with CML undergoing imatinib treatment. There is a significant (p = 0.011) decline in bone resorption over time, as indicated by urine desoxypyridinolin (DPD). However, in most cases, values are still in the lower normal range. Bone formation as indicated by osteocalcin levels is impaired, with most values below the lower normal range and exhibiting a borderline significant (p = 0.041) decline over time.44
Alteration of bone biochemical markers in children with CML undergoing imatinib treatment. There is a significant (p = 0.011) decline in bone resorption over time, as indicated by urine desoxypyridinolin (DPD). However, in most cases, values are still in the lower normal range. Bone formation as indicated by osteocalcin levels is impaired, with most values below the lower normal range and exhibiting a borderline significant (p = 0.041) decline over time.44
Treatment Response
Data in approximately 150 children with CML treated with first-line imatinib have shown that in CML-CP, 96% achieve a CHR and 69% a CCyR after 1 year.22–24,42,44 However, it is likely that there is a large pool of uncollected data because the majority of children receive imatinib without being included in formalized trials. The Children's Oncology Group was the first to report a phase I study of 31 children and adolescents who received imatinib after IFN failure.22 CHR occurred in all children in CML-CP, and 10 of 12 (83%) evaluable children achieved CCyR (although this response was later lost in two patients). Responses were also seen in the more advanced phases but were not persistent. Thirty children from eight European countries were enrolled in a phase II study.23 Imatinib-induced CHR occurred in 80% and CCyR in 12 of 20 (60%) children included in CML-CP and in 2 of 7 (29%) included in CML-AP. A reduction in the ratio of BCR-ABL to ABL to under 0.01 was achieved in 11 (50%) children. Forty-four children with de novo CML-CP were enrolled in a French phase IV study with a median treatment duration of 16 months (range, 1–67 months).42 The CHR rate was 86% and 98% at 3 months and 6 months, respectively, while 62% of patients achieved CCyR at 12 months. The proportion of major molecular response (MMolR) was 34% at 12 months. Similar response rates were described in 51 patients enrolled in a German trial called CML-Paed II.44 A landmark analysis of patients entering in CML-CP showed that 2 of 42 patients (5%) had no CHR at month 3, 2 of 28 (7%) had no CCyR at month 12, and 2 of 19 (15%) patients achieved no MMolR at month 18 after starting imatinib. There is no doubt that the majority of pediatric patients who start treatment in CML-CP respond at least as well to TKI as adults. Whether the kinetics of the response rates differ in an age-dependent manner still remains to be determined. There seems to be a trend for inferior response rates in both adult and pediatric patients if they present with an e13a2 type of the BCR-ABL transcript compared with the e14a2 transcript type.45,46
Imatinib Failure Due to Resistance or Intolerance
Failure of imatinib primarily comprises resistance to the drug or drug intolerance. According to the time of onset, resistance to imatinib can be categorized into primary resistance, a lack of efficacy from the onset of treatment, and secondary (acquired) resistance (relapse), an initial response followed by a loss of efficacy with time. For a more detailed discussion with respect to resistance (hematologic, cytogenetic, molecular) in the different phases of CML, the reader is referred to Hochhaus et al..47 Resistance may be multifactorial, including BCR-ABL mutations of the kinase domain interfering with imatinib binding, BCR-ABL amplification or overexpression, clonal evolution and decreased imatinib bioavailability, or cell exposure.48 Clonal evolution and mutations are the most important factors and are related to each other.49 It is noteworthy that survival remains stage dependent, and that both dasatinib and nilotinib are ineffective against mutation T351I BCR-ABL.49 Patients who cannot continue taking imatinib due to adverse effects, either hematologic or non-hematologic, are regarded as intolerant. Although the number of pediatric patients is still very small, such children with non-hematologic intolerance experience no cross-toxicity when exposed to dasatinib; however, patients whose intolerance was due to cytopenia (neutropenia or thrombocytopenia or both) may experience analogous cytopenias with dasatinib (authors' experience in five pediatric patients; data not yet published). From the results of a small series, it can be deduced that the rate of imatinib failure in pediatric CML-CP treated up-front with imatinib is in the range of 10% to 20% of all patients (8 of 44 in the French trial and 6 of 47 in the German trial).42,44 This compares well to adults as in the IRIS trial, 18% of whom exhibited secondary resistance after a median of 5 years.6 For children who fail or progress, there are presently four treatment options: 1) increasing the dosage of imatinib, 2) changing to dasatinib, 3) allo-SCT, or 4) treatment of CML with well-established drugs such as IFN-α or hydroxyurea.34
Increasing the Dosage of Imatinib
While it is easy to increase the dose of imatinib in resistance, there is no data from controlled trials in children as to whether this approach is effective. Noncompliance, especially in teenagers, should be ruled out by determining the drug serum level before any dose modifications are undertaken. Patients who fail imatinib treatment should be screened for the presence of kinase domain mutations, especially for T351I, which excludes ongoing TKI treatment. In adults, some patients will not achieve a worthwhile response to the higher dose, and the majority of those responding in the short term will gradually lose their initially good response.49,50
Changing to Dasatinib
Two studies of the use of dasatinib in children and adolescents have been presented at international meetings. Preliminary data showed that there were two CHRs and three partial responses in 5 of 6 response-evaluable children with CML.51 An ongoing Bristol-Myers Squibb-sponsored phase I/II study of dasatinib (CA180–18) has entered 20 children with CML (12 in CML-CP and 8 in more advanced phases). The response in CML-CP was excellent, displaying CHR in 10 of 12 children and CCyR in 7 of 12 children on 60 to 80 mg/m2/d, while 62% of patients with Ph+ ALL, CML-AP, or CML-BP achieved MCyR. Dasatinib was well tolerated up to a dose level of 120 mg/m2, and treatment-related toxicities were mostly mild to moderate in severity (grade 4 or less), with nausea and diarrhea occurring most frequently. Pharmacokinetics suggest that the half-life of dasatinib of approximately 2 h in patients under the age of 20 years may be somewhat shorter compared with the more than 3 h in adults. Thus, changing the treatment to dasatinib in case of imatinib failure is probably the best option (M. Zwaan, personal communication). In adults, it appears that about 50% of those who fail imatinib due to resistance, and a slightly higher proportion of those who fail due to intolerance, will obtain longer-term benefit from switching to either nilotinib or dasatinib.48 In young patients, there are not yet any data available on the use of nilotinib, so there is an urgent need for well-designed clinical trials in this patient population.
Allo-SCT
Allo-SCT has become a form of rescue treatment for those infrequent imatinib-resistant cases. In most circumstances, early signs for disease progression can be detected appropriately by adequate screening based on strict monitoring. Because a search for a matched voluntary unrelated donor can often take several months, donor searches immediately after diagnosis are probably justified for patients who would be considered potential allograft candidates if they develop resistance to medical therapy. Allo-SCT is indicated ideally before clinical or hematological signs of progression to CML-AP or CML-BP are apparent. Whether such patients should have an initial trial of a second-generation TKI or go straight to allo-SCT is still not clear. Allo-SCT in CML-BP induces a durable remission in at best 20% of patients. In these patients, therefore, any attempt must be undertaken before allo-SCT to achieve at least a hematological response—or even better a MCyR—by chemotherapy and/or treatment with TKI.34,48 Because the remission tends to be short, it is appropriate to proceed as early as possible to allo-SCT, even if this means accepting not fully matched donors. Central nervous system prophylaxis is also advisable. It should be remembered that patients treated successfully for CML-BP, unlike patients treated with imatinib in first CML-CP, may proceed from complete molecular response (CMolR) to overt relapse very rapidly; therefore, to be useful, ongoing PCR monitoring must be carried out at much more frequent intervals than for patients treated in CML-CP. From a theoretical standpoint, reduced-intensity conditioning (RIC) is also an attractive treatment approach in younger patients because it might preserve fertility and reduce toxicity; however, data relating to its use in children and adolescents are scant and as yet uninformative.52 It must be kept in mind that the effect of RIC is based on an allo-immune response, which, in comparison with other leukemias, has been proven to work more successfully in CML. However, RIC in CML is associated with a high risk of relapse, and therefore is unlikely to cure advanced phase of the disease. Monitoring of MRD at short time intervals is obligatory, and donor lymphocyte infusions and /or TKI have to be considered for treating disease recurrence at an early stage. For patients who have never been treated with imatinib or for those being transplanted early in the course of the disease after having achieved a MMolR, RIC might be an option. However, currently most children undergo allo-SCT because of imatinib failure. Careful analysis of resistance to TKI prior to RIC aims at identifying cases that are refractory even to second-generation TKI. For the latter group, donor lymphocyte infusions in the first year after allo-SCT bears a high risk of graft-versus-host disease (GVHD), thereby turning RIC into a less rational procedure.
Treatment with Other Drugs
Perhaps the most difficult clinical challenge is how to treat the patient who has clearly failed dasatinib yet is lacking a donor for allo-SCT. Non-allo-SCT options include the use of IFN-α or hydroxy-urea, neither of which can be expected to prolong life any longer than what has been achieved with this approach in the past millennium. However, this treatment may buy some time until new agents become available for children that are now being tested in ongoing phase II trials in adults. In addition, one must remember that patients who have failed two different TKIs have defined themselves as having a relatively aggressive form of CML. Drugs targeting BCR-ABL on domains other than the ATP-binding site (RAS, MTDR pathways, HSP90 molecular chaperone machinery, dual SRC/ABL inhibitors) are very promising, yet available in controlled trials for adults only. However, individual pediatric cases might profit from compassionate use.53 In addition, all forms of alternative donor allo-SCT (mismatched cord blood or haplo-identical procedures) can be considered when other alternatives are not available in therapy-resistant patients.
Therapy of Relapse after Allo-SCT
A relapse risk as high as 20% has been reported after allo-SCT for CML-CP.11,15–17,19 Therefore follow-up examinations must include close monitoring for MRD. Whether the use of imatinib as post-allo-SCT maintenance therapy helps in preventing relapse remains uncertain for both the adult and the pediatric cohort and awaits future prospective studies.54 It may be beneficial for patients in whom a high relapse risk is anticipated, such as in cases of T-cell-depleted allo-SCT. For patients experiencing molecular, cytogenetic, or hematological relapse post allo-SCT, the choice of therapy is controversial, with both donor lymphocyte infusions and imatinib having demonstrated the potential to achieve molecular remission.31,34,55,56 Also, donor lymphocyte infusions might induce life-threatening GVHD, especially if administered early after allo-SCT. In most cases in which another remission could be induced by imatinib, it is commonly followed by another relapse once the drug is withdrawn. However, imatinib should be used first, especially if the relapse occurs within 1 year of transplant and/or if the child has active GVHD. As indicated by the results of MRD monitoring, this might be followed by the cautious use of low-dose donor lymphocyte infusions, with subsequent dose escalation for lack of response as clinically indicated.56 It is also possible that a child who relapses post-transplant for imatinib failure may benefit long-term from dasatinib.31
Arguments for and against Allo-SCT and Timing of the Procedure
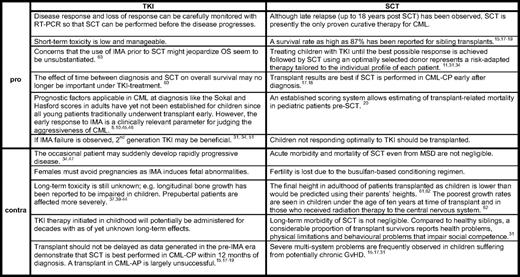
While allo-SCT is undoubtedly the preferred second- or third-line treatment for CML-CP in adults, in children the optimal timing is still the subject of ongoing debate. The shift away from allo-SCT toward imatinib monotherapy, even with a matched sibling donor available, has yet to be globally accepted. In some countries with reduced financial health resources, cost considerations favor allo-SCT as a “once-only” procedure as opposed to a lifelong treatment with an expensive drug. In a recent survey addressing treatment approaches for CML in pediatric centers in first-world countries such as the United States and Canada, there was universal agreement for using imatinib as a front-line treatment.57 However, allo-SCT in CML-CP1 was recommended by 17 of 27 (63%) responding physicians when a matched sibling donor was available as part of the initial therapy. The timing of transplant was also judged as important, and allo-SCT within 1 year of diagnosis, regardless of donor source, was suggested. In the German clinical trial CML-Paed II, treatment with allo-SCT was the recommended therapeutic option after a 2-year period of reducing the leukemia cell load with imatinib.11 However, none of the first 12 patients in the trial who had been treated during this period and achieved at least MMolR opted for allo-SCT.44 Major drawbacks of allo-SCT are the considerable related mortality associated with the procedure and, in survivors, long-term morbidities including infertility and endocrine deficiencies are not inconsequential. On the other hand, TKIs may significantly affect growth, and females having to take TKI for the rest of their lives are not supposed to become pregnant because of possible adverse effects on the fetus. Table 2 aims at balancing the arguments for and against each of the two treatment options for patients in CML-CP. In advanced phases of CML, however, allo-SCT is the first choice of treatment after another hematologic or better cytogenetic response has been achieved.
Stopping Imatinib and ‘Cure’ of CML
When and if imatinib can safely be stopped in a patient who is responding to it is a pressing question, particularly for children facing an unknown burden of side effects with potentially lifelong treatment. Recently, a pilot study was updated reporting on the first adult patients who discontinued imatinib for different reasons after having achieved a CMolR lasting more than 2 years.58 Half of the patients relapsed, mostly within less than 6 months after discontinuation, but molecular remission could be regained by reinstitution of imatinib. With an impressively long follow-up interval of 42 months (median) after discontinuation, the other half of the patients maintained their CMolR. The INTERIM (Study of Intermittent Imatinib Treatment) trial of 82 elderly patients who achieved a sustained CCyR investigated whether intermittent imatinib treatment (1 month on/1 month off) would result in maintaining the drug response.59 With a rather short follow-up of 6 months, 87% maintained a MMolR, demonstrating that intermittent dosing of imatinib does not show a negative trend. The finding that a small proportion of patients with CML-CP who have perfectly responded to TKI treatment could safely stop treatment for an as-yet-unknown period remains a challenge. Trials in children might explore whether intermittent dosing of imatinib can have a positive role on their longitudinal growth development and on other side effects. As a word of caution, stopping imatinib in situations of undetectable or low BCR-ABL transcripts in patients who are not enrolled in a clinical study can currently not be recommended.
Disclosures
Conflict-of-interest disclosure: MS has received research funding from Novartis and has consulted for Brystol-Meyers Squibb and Wyeth.
Off-label drug use: None disclosed.
Correspondence
Prof. Dr. med. Meinolf Suttorp, Division of Pediatric Hematology and Oncology, Department of Pediatrics, University Hospital Carl Gustav Carus, Fetscherstr. 74, D-01307 Dresden, Germany; Phone: 49-351-458-3522; Fax: 49-351-458-5864; e-mail: meinolf.suttorp@uniklinikum-dresden.de