Abstract
Important studies challenging previous approaches to the treatment of adults with Philadelphia chromosome–negative acute lymphoblastic leukemia (ALL) have emerged in the past decade. Donor versus no donor comparisons of allogeneic transplant highlight a potent graft-versus-leukemia effect in ALL, and the application of reduced-intensity conditioning transplants may exploit this effect while reducing non-relapse mortality. The adoption of the use of pediatric intensity-type regimens in adolescents and young adults shows promise to improve outcomes in this population. New therapeutic targets such as mutations in NOTCH1 in T-cell ALL and CD22 in pre-B ALL are being exploited in clinical trials. The application of molecular techniques and flow cytometry to quantitate minimal residual disease will allow further stratification of patients by risk. Although the outcomes of adults with ALL lag behind the stunningly successful results seen in children, new paradigms and new discoveries bring hope that this disparity will steadily lessen.
In the first decade of the new millennium, multiple studies have begun to change our thinking about the treatment of adults with acute lymphoblastic leukemia (ALL). In pediatric patients cure rates in the range of 80% to 90% are now attainable.1 While adult patients with ALL now have a 90% chance of entering first complete remission (CR) with modern chemotherapy, most patients will relapse, and leukemia-free survival with 3 to 7 years of follow-up in large series is only in the range of 30% to 40%.2 The poor outcome of chemotherapy in adults with ALL as compared to children relates to multiple factors, including poor tolerance of intensive courses of chemotherapy and a higher incidence of poor prognostic subtypes of ALL such as Philadelphia chromosome–positive ALL and a lower incidence of favorable subtypes such as the t(12;21).
Evolving paradigms in the treatment of adult ALL include the application of intense pediatric regimens to the treatment of adolescents and young adults and the increasingly recognized importance of the graft-versus-leukemia effect in the cure of patients. The identification of new molecular abnormalities as treatment targets, such as NOTCH1, bring the hope that new agents directed at these pathogenic mechanisms may improve outcomes. The application of minimal residual disease testing will allow for better stratification of patients and tailored therapy based on risk. These new paradigms bring hope for improved outcomes, but currently are also leaving clinicians with increased uncertainty about how to optimally manage adult patients with newly diagnosed ALL.
Graft-Versus-Leukemia Effect in ALL
While most transplant clinicians have, in recent years, considered the allogeneic graft-versus-leukemia effect to be weak in ALL on the basis of poor results with donor lymphocyte infusions in the relapse setting, the overall poor prognosis of adults with ALL led investigators to explore the use of allogeneic and autologous bone marrow transplantation compared with chemotherapy in randomized trials beginning in the late 1980s and early 1990s.
In the LALA-87 trial, patients over the age of 50 were treated with chemotherapy only, while those under the age of 40 with an HLA-identical sibling received an allogeneic SCT.3 The remaining patients were randomly assigned to receive either autologous transplant or chemotherapy. This trial demonstrated similar disease-free survival between the autologous transplant and chemotherapy patients. For the allogeneic patients, those with high-risk features including the presence of BCR-ABL1 gene rearrangement, older age, elevated white count, or delay in achieving remission had a superior outcome to those patients receiving chemotherapy or autologous transplantation.4 In the LALA-94 trial patients with high-risk ALL were allocated to allogeneic bone marrow transplantation (BMT) if they had an HLA-identical sibling or were randomized to autologous BMT or chemotherapy if they did not. Disease-free survival was 45% in patients with a donor versus 18% in those without (P = .007).5
A meta-analysis conducted in the 1990s of these studies and others, totaling 7 studies encompassing 1274 patients, carried out a donor versus no donor comparison and showed a survival advantage for patients with a donor and that this survival advantage was even greater in the subset of high-risk patients. No beneficial effect of autologous BMT was noted.6 On the basis of studies such as these an evidence-based review of the role of BMT in the treatment of ALL recommended allogeneic BMT for ALL in first remission for patients with high risk, but not standard risk, disease.7
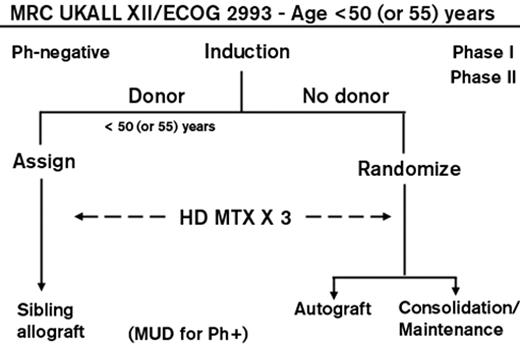
A trial carried out by the Medical Research Council in Great Britain and the Eastern Cooperative Oncology Group in the United States, the largest ever reported, treated nearly 2000 patients. The treatment consisted of 2 months of induction chemotherapy; those achieving a complete remission were allocated to allogeneic BMT if they had an HLA-matched sibling or, if not, were randomized to receive an autologous BMT or consolidation and maintenance chemotherapy (Figure 1 ). Those with Philadelphia chromosome–positive ALL were allowed to receive an allogeneic transplant from an unrelated donor and subsequently, with its availability, were also treated with imatinib. High-risk patients were defined as patients older than 35 years or those with a high WBC count at presentation ( ≥ 100 × 109/L for B lineage and ≥ 30 × 109/L for T lineage) along with all patients with the Philadelphia chromosome. In a donor-versus-no donor comparison for the Philadelphia chromosome–negative patients, a potent graft-versus-leukemia effect was found in all patients with a 5-year improved overall survival (OS) of 53% versus 45% (P = .01). This overall survival advantage was also seen in the standard-risk patients where 62% of those with a donor versus 52% of those without a donor were alive at 5 years (P = .02). Surprisingly, benefit for transplant in the donor group was not seen for the high-risk patients with a 5-year OS of 41% versus 35% of high-risk patients with no donor (P = .2). A high treatment-related mortality of nearly 36% at 2 years mitigated the benefit of a significantly lower relapse rate.8 Thus, this large study suggested that standard-risk adults with ALL could benefit from a graft-versus-leukemia effect.
Recently, a similar report from the Netherlands of 288 patients under the age of 55 from two consecutive prospective studies described a donor-no donor comparison between patients with a compatible sibling donor who underwent allogenic transplant versus other patients who underwent an autologous transplant. In the donor group disease-free survival at 5 years was significantly better at 60% compared with 42% in the no-donor group (P = .01). This improved outcome was more pronounced in the standard-risk patients with a donor who had an OS at 5 years of 69% (hazard ratio = 0.47; 95% CI 0.26–0.84; P = .007).9
Reduced-intensity conditioning (RIC) regimens are beginning to be utilized in the treatment of patients with ALL to overcome some of the increased incidence of treatment-related mortality associated with myeloablative allogeneic BMT. The European Group for Blood and Marrow Transplantation (EBMT) has reported one of the largest series to date. In it, the outcomes of 97 adult patients with ALL, including one third of the patients in first CR with the majority in higher levels of CR or with refractory or persistent disease. The patients received a variety of RIC regimens and, with nearly 3 years of follow-up, the OS for the first CR patients was 52%. OS was 27% and 20%, respectively, in patients in second/third CR or with a more advanced disease.10 In a retrospective comparison of RIC transplantation compared with myeloablative (MA) transplantation from the EBMT, 97 patients receiving a RIC transplant were compared with 504 patients receiving a MA transplant. Patients in the RIC group were older. Non-relapse mortality at 2 years was lower in the RIC group at 22% compared with 32% for the MA group, but relapse incidence was higher in the RIC group; and the overall leukemia-free survival was similar at 38% for MA and 37% for RIC. These results are promising given that the RIC patients were older and likely had more comorbidities.11 Thus, these recent studies have confirmed that, contrary to prior beliefs, a potent graft-versus-leukemia effect exists in ALL and has a role in both standard and high-risk patients. This topic is thoroughly reviewed at another session of this meeting.12
Treatment of Adolescents and Young Adults
A surprising finding in the therapy of ALL in children has been that much of the success in the past several decades has been a result of altering the doses and schedules of existing drugs and not as a result of the introduction of major new drugs. Adult regimens are typically less intense than pediatric regimens. The Cancer and Leukemia Group B (CALGB) and the Children’s Cancer Group (CCG) first asked whether adolescents and young adults (AYA) between the ages of 16 and 20 fared differently whether they were treated on CALGB protocols or CCG protocols. The results of this study, first reported in 2000 and subsequently published in 2008, demonstrated that although the complete remission rates were identical for the AYAs treated on the CALGB and CCG trials, the AYAs had a 63% event-free survival (EFS) and 67% OS at 7 years on the CCG trials compared with 34% and 46%, respectively, on the CALGB trials. Although the age range of the patients treated were the same in both studies, the median age of the patients in the CALGB studies were 19 years as compared with 16 years on the CCG trials. A puzzling finding from this study was that the EFS for the 16 to 17 year olds on the CALGB studies were similar to the same age group on the CCG studies (55% vs 64%, respectively, P = .49). The inferior outcome for the CALGB patients was confined to the 18 to 20 year olds whose EFS was only 29% as compared with 57% for the 18 to 20 year olds treated on the CCG studies (P = .01). The CCG patients received intensive and earlier central nervous system prophylaxis, which resulted in a significantly lower incidence of isolated central nervous system relapses. Also the doses of non-myelosuppressive drugs such as corticosteroids, vincristine, and L-asparaginase were much higher in the CCG regimens compared with the CALGB regimens.13 This increased intensity of non-myelosuppressive drugs was particularly evident in post-remission therapy.
Since the initial report of these findings in 2000, multiple national European cooperative groups have reported similar results with improvement in the outcome of AYAs treated on pediatric protocols as compared to adult protocols14–19 (Table 1 ). However, these trials are difficult to interpret because of the differing ages of the patients studied, small numbers of patients, variations in the regimens utilized and the varying application of BMT in different studies. The median age of the adolescents on the adult studies are usually at least 2 years older than the adolescents on the pediatric studies.
In the study from Finland a group of 128 patients aged 10 to 16 years (median age 12.9 years) treated on pediatric protocols were compared with 97 patients aged 17 to 25 years (median age 18.9 years). Despite this significant age discrepancy and differences in the dosing of various drugs in the two groups, the complete remission rates were the same (96% pediatric, 97% adult) and there was no significant difference in the 5-year EFS (67% pediatric, 60% adult; P = n.s.) or OS (77% pediatric, 70% adult; P = n.s.) between the two groups.19
Whether these improved outcomes are solely a result of the higher doses of non-myelosuppressive drugs or whether they relate to other factors such as the greater experience of pediatric hematologists in caring for adolescents with ALL compared with adult hematologists-oncologists, or stricter adherence to scheduled treatments is unclear.20 It also appears that adults do not tolerate drugs utilized in ALL treatment as well as children do.21
These results have prompted new studies where pediatric ALL regimens have been adapted to the treatment of younger adults. With short follow-up, these reports suggest EFS and OS outcomes in the range of 60%.22,23
The GRAALL-2003 was a phase II study where patients between the ages of 15 and 60 were eligible to receive a “pediatric-inspired therapy” that contained higher doses of asparaginase, vincristine and prednisone compared with the group’s previous adult protocols. With a median follow-up of 42 months the EFS and OS were 55% and 60%, respectively. Age was an important factor in outcome. The authors identified a best age cutoff of 45 years, with patients over age 45 having a chemotherapy-induced death rate of 23% as compared with 5% in younger patients, with EFS of 58% and 46% respectively (P = .03). The authors do not specify if there was any difference in outcome by age within the group of patients 15 to 45 years old.23
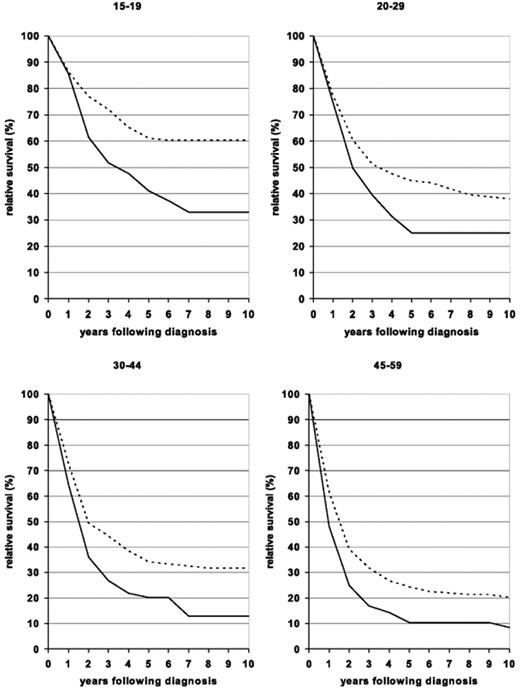
These results are similar to what has been seen with allogeneic BMT in younger patients, but because the follow-up is shorter, it is unclear as yet whether these encouraging outcomes will remain durable over time. Thus, these studies, while encouraging, are not yet definitive proof of the benefit of this approach. In fact, a study recently reported in abstract form suggested that the results of the hyper-CVAD regimen are improved in younger adults with EFS and OS rates of 55% and 67%, respectively.24 Interestingly, recent data from the Surveillance, Epidemiology, and End Result (SEER) database suggest that outcomes for all adults between the ages of 15 and 60 have improved over time. In those over age 60, similar improvement has not been seen (Figure 2 ).25
While a trial randomizing AYAs to a pediatric regimen versus an adult regimen will likely not be done, an innovative phase II comparison is being conducted in the United States where one arm of a high-risk pediatric ALL regimen (protocol AALL0232) will be compared to a group of AYAs up to the age of 30 treated with the identical regimen in an adult intergroup protocol (C10403). In addition to assessing the outcomes of AYAs treated on both these regimens, adherence to the two regimens in terms of dosing and schedule will be closely monitored and will provide more definitive proof as to whether the pediatric regimen can bring similar results in AYAs treated by adult hematologists-oncologists. A similar trial is being conducted by the EORTC and Dutch HOVON groups. The results of these trials are eagerly awaited.
New Targets and Mechanisms of Disease
Cytogenetic abnormalities related to prognosis have been evident in patients with ALL for many years and remain one of the strongest prognostic factors for outcome.26 These studies have demonstrated the range of cytogenetic abnormalities found in patients with ALL but also highlighted the difference in the incidence of these abnormalities between children and adults. They show that these poor prognostic cytogenetic abnormalities are more frequently seen in adults with ALL and the incidence of these adverse prognostic abnormalities increases as patients age. Cytogenetic abnormalities remain the strongest predictor of treatment outcome in patients with ALL as in AML.26
The identification of recurring cytogenetic abnormalities in patients with ALL has led to the identification of the genes and gene products juxtaposed as a result of these translocations. A prominent abnormality in this regard is TEL-AML-1, the gene that results from a translocation between chromosomes 12 and 21. Patients with more than 50 chromosomes in their leukemic cells (hyperdiploidy) and patients with the TEL-AML-1 gene abnormality represent nearly 50% of all cases of pediatric ALL. These two abnormalities are associated with a good prognosis.1
New genetic abnormalities continue to be identified as increasingly sophisticated molecular techniques become available. Some of these include intrachromosomal amplification of chromosome 21, chromosome 9p deletions, and mutations in the FLT3, PTPN11, and RAS genes. These are summarized in a recent review by Meijerink.27 Of increasing interest is the use of high-resolution genome-wide platforms for the detection of regions of loss of heterozygosity and DNA copy number abnormalities. These studies are beginning to unravel the ways in which the multiple genetic and molecular legions identified in ALL may cooperate to produce the disease. In a key study, Mullighan and colleagues performed a genome-wide analysis of 242 pediatric ALL samples utilizing single-nucleotide polymorphism (SNP) arrays and genomic DNA sequencing. In precursor B ALL, they identified structural rearrangements, point mutations, deletions, and amplifications in genes encoding the main regulators of B-lymphocyte development and differentiation in 40% of cases. The PAX5 gene was altered in 32% of cases.28 These molecular techniques are highlighting abnormalities such as loss of heterozygosity (LOH), copy number abnormalities (CNA), and epigenetic modifications including histone modification and cytosine methylation.
The most interesting discovery in recent years in T-cell ALL has been the finding of activating mutations in the NOTCH1 and FBXW7 genes in more than 50% of cases.29
These interesting developments are described in more detail in the report by Ferrando30 in this volume.
The development of new chemotherapeutic and biologic agents for other diseases have begun to find application in the treatment of ALL. The remarkable success of rituximab in the treatment of non-Hodgkin’s lymphoma and the known negative prognostic significance of CD20 expression in ALL31 led to the exploration of rituximab in precursor-B ALL and has suggested that rituximab can improve outcome when combined with hyper-CVAD compared with patients receiving hyper-CVAD alone.32 Randomized trials assessing the role of rituximab in precursor-B ALL are in progress.
CD22 is a B lymphocyte–restricted 135-kDa transmembrane sialoglycoprotein member of the immunoglobulin super-family that is initially present in the cytoplasm of developing B cells. Surface expression of CD22 occurs at later stages of B-cell development. It is present on 60% to 80% of B-cell malignancies.33 Epratuzumab is a humanized anti-human CD22 antibody that rapidly internalizes into the cell upon binding to CD22. Epratuzumab has single-agent activity in diffuse, large, B-cell lymphoma.34 It has been combined with chemotherapy and rituximab in the treatment of newly diagnosed diffuse large B-cell lymphoma with promising results.35 In ALL, studies are indicating that it can be combined with ALL-type therapy with encouraging results.36
An interesting agent in the treatment of T-cell ALL is nelarabine, a pro-drug of guanine arabinoside. Promising results have been seen in pediatric patients in the relapsed and refractory setting.37 In adults with relapsed or refractory T-cell ALL a recent study has shown that a regimen of 1.5 g/m2/day on days 1, 3, and 5 can safely be administered in multiple cycles every 22 days. It produced a complete remission rate of 31% with an overall response rate of 41% in a group of high-risk patients. The principal toxicities were cytopenias, with only one grade 4 adverse event in the nervous system.38
A number of other promising agents are in development for ALL and are listed in Table 2 .
Minimal Residual Disease
Although almost all patients with newly diagnosed ALL achieve morphologic and cytogenetic complete remission, some patients with pediatric ALL and many patients with adult ALL relapse. This indicates that many of these patients have undetectable residual disease at the time of achievement of a complete remission. The introduction of sensitive molecular techniques utilizing clonal immunoglobulin or T-cell receptor gene rearrangements has been effectively utilized to detect minimal residual disease. In pediatric ALL it was demonstrated over 10 years ago that the presence of residual disease at the end of induction chemotherapy or at later time points was a powerful predictor of relapse, independent of other risk factors.39 The challenge of utilizing immunoglobulin or T-cell receptor gene rearrangement studies is that each patient’s gene rearrangement is unique and must be established for each patient. The increasing sophistication of flow cytometry has also led to wider use of this technique to identify distinct immunophenotypes in patients that allow the detection of one leukemic cell amongst 10,000 normal cells.40 The German Multi-Center Study Group for adult ALL has published data indicating that patients who have a rapid decline in their minimal residual disease (MRD) measurement within the first month of therapy had a 3-year relapse rate of 0%. Another subset of patients who had MRD detectable until week 16 of therapy had a 3-year relapse rate of 94%. Patients in between these two groups had an intermediate risk of 47%.41 The patients with a rapid decline in their MRD represented only 10% of the patients studied, highlighting another reason for the poorer prognosis of patients with adult ALL. A study from Italy has recently confirmed the value of MRD determinations in determining outcome of patients with adult ALL.42 In adult ALL, MRD measurement has not yet become the standard of care in all centers but will likely become so in the future. An excellent summary of the state of MRD measurement in ALL has recently been published.43
Conclusion
The successes achieved in the treatment of pediatric ALL in the past 40 years have and will continue to influence the treatment of adults with ALL. The use of pediatric regimens in the treatment of young adults may enhance their outcome, but further study is needed to determine the long-term results of this approach and whether adults can truly tolerate these intensified regimens in terms of toxicity. Advances in allogeneic BMT hold the promise that a reduced-intensity conditioning approach can exploit the graft-versus-leukemia effect while reducing toxicity. These studies are exciting, but have also increased uncertainty in the minds of clinicians treating adults with ALL as to the optimal approach. It is imperative that patients enter clinical trials so these new approaches can be carefully studied. New immunotherapeutic and chemotherapeutic agents will enhance the therapeutic armamentarium for the treatment of this difficult disease. The identification of new molecular abnormalities will enhance our prognostic abilities and allow for the identification of new drugs. Minimal residual disease testing is and will continue to stratify patients for risk of relapse and allow further individualization of therapy.
Overall schema for the international ALL trial conducted by the MRC and ECOG. Reprinted with permission from Rowe JM. Optimal management of adults with ALL. Br J Haematol. 2009;144:468–483.44
Overall schema for the international ALL trial conducted by the MRC and ECOG. Reprinted with permission from Rowe JM. Optimal management of adults with ALL. Br J Haematol. 2009;144:468–483.44
Ten-year relative survival curves of patients with ALL by major age groups. Period estimates for 1980–1984 (solid curves) and 2000–2004 (dashed curves). Reprinted with permission from Pulte D, Gondos A, Brenner H. Improvement in survival in younger patients with acute lymphoblastic leukemia from the 1980s to the early 21st century. Blood. 2009;113:1408–1411.25
Ten-year relative survival curves of patients with ALL by major age groups. Period estimates for 1980–1984 (solid curves) and 2000–2004 (dashed curves). Reprinted with permission from Pulte D, Gondos A, Brenner H. Improvement in survival in younger patients with acute lymphoblastic leukemia from the 1980s to the early 21st century. Blood. 2009;113:1408–1411.25
Disclosures Conflict-of-interest disclosure: The author received honoraria and research funding from Enzon. Off-label drug use: Rituximab treatment for acute lymphoblastic leukemia.
References
Author notes
Division of Hematology, Mayo Clinic, Rochester, MN