Abstract
The treatment and medical management of aplastic anemia fundamentally differ between patients with inherited versus acquired marrow failure; however, the diagnosis of an inherited bone marrow failure syndrome is frequently obscure. Recent exciting advances in our understanding of the molecular pathophysiology of the inherited bone marrow failure syndromes have resulted in a profusion of new tests to aid in diagnosis. This in turn has raised questions regarding the appropriate choice of testing for the patient presenting with aplastic anemia. Important clues to the diagnosis of an inherited marrow failure syndrome may be gleaned from careful attention to the clinical history, physical exam, and laboratory workup.
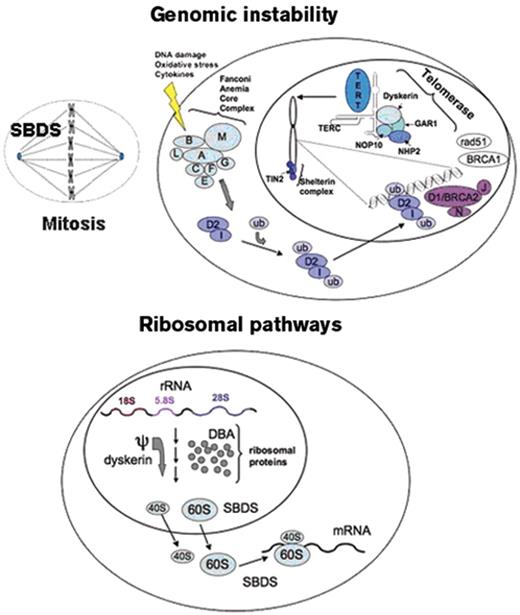
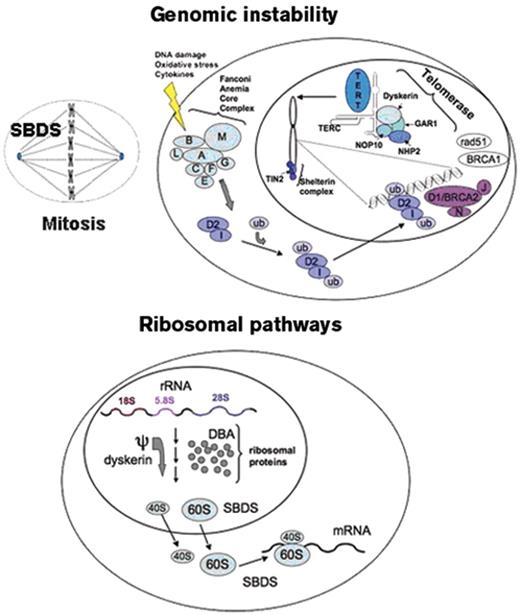
Many patients with inherited bone marrow failure syndromes (IBMFS) first present to the hematologist with aplastic anemia. These IBMFS include Fanconi anemia, dyskeratosis congenita, Shwachman-Diamond syndrome, congenital amegakaryocytic thrombocytopenia, and, rarely, Diamond-Blackfan anemia. The distinction between the IBMFS and acquired aplastic anemia is critical to inform choice of therapy and to guide medical management. The recognition of the IBMFS is more difficult than previously acknowledged. The clinical spectrum of the inherited marrow failure syndromes is broader than the early literature descriptions, and classical clinical findings may be subtle or absent. Furthermore, the inherited marrow failure syndromes are no longer the exclusive domain of the pediatric hematologist: an increasing number of these patients first come to medical attention well into adulthood. Indeed the prevalence of the inherited marrow failure syndromes is poorly defined and previously underestimated. Aplastic anemia, myelodysplastic syndrome (MDS), or even leukemia may be the presenting manifestation of an inherited marrow failure syndrome. The rapid proliferation of available laboratory tests has greatly advanced our ability to diagnose these syndromes but has also created confusion regarding their indications and interpretation. This article will provide an overview of potential clues to the diagnosis of an underlying inherited marrow failure syndrome and current laboratory diagnostic tests available to screen for the IBMFS. These syndromes have provided important insights into the molecular pathways contributing to marrow failure and malignant transformation (Figure 1 ).
Clinical implications of the IBMFS
The diagnosis of an inherited marrow failure syndrome carries profound implications for choice of therapy and medical management. Standard hematopoietic stem cell transplant regimens for aplastic anemia are associated with increased transplant regimen-related toxicities for patients with certain inherited marrow failure syndromes such as Fanconi anemia, dyskeratosis congenita, and Shwachman-Diamond syndrome.1 Patients with these IBMFS require specialized reduced-intensity transplant regimens to avoid excessive morbidity and mortality. Furthermore, stem cell transplantation treats only the marrow disease; the other organ system abnormalities, including predisposition to solid tumors, remain. Patients must be monitored carefully for malignancies since early detection offers the best opportunities for cure. In some cases, toxicities of hematopoietic stem cell transplantation (HSCT) regimens may exacerbate the other organ system diseases.2 As with any rare disorder, HSCT on investigational protocols specific for each IBMFS is recommended to improve therapy. The diagnosis of an IBMFS must be ruled out in all potential sibling donors of a proband. Aplastic anemia in patients with IBMFS responds poorly to treatment with antithymocyte globulin and cyclosporine, a standard medical therapy for the patients with acquired marrow failure. Marrow failure in patients with Fanconi anemia or dyskeratosis congenita may improve upon treatment with androgens such as oxymetholone.3,4
The early diagnosis of an IBMFS also allows for family planning. Some families interested in having additional children decide to seek prenatal testing or preimplantation genetic diagnosis. Diagnosis also permits carrier testing for family members seeking genetic counseling. As noted above, testing of siblings for IBMFS informs donor selection to avoid choosing an affected donor. Medical evaluation and diagnostic testing of family members, particularly siblings, of the affected proband is imperative since family members sharing the same mutation in an IBMFS gene can manifest widely variant phenotypes and severity of symptoms.
Clinical Evaluation of the Marrow Failure Patient
Clinical History
A thorough clinical history may reveal important clues raising suspicion for an inherited marrow failure syndrome. Although many findings are non-specific in isolation, they may warrant careful consideration in the context of a patient with marrow failure (Table 1 ).
Birth history may reveal complications such as unexplained intrauterine growth retardation or small birth weight. Short stature or failure to thrive is a common feature of the IBMFS, though normal growth does not rule out an inherited marrow failure syndrome. A history of a skin rash, fine thin dysmorphic nails, poor nail or hair growth, early hair graying or early hair loss, dental anomalies such as dysmorphic teeth, enamel hypoplasia, or oral leukoplakia (easily mistaken for thrush) may suggest dyskeratosis congenita, although marrow failure may precede these findings.5 Anomalies of the eyes and ears have been described for Fanconi anemia and exudative retinopathy may accompany dyskeratosis congenita (formerly part of Revesz syndrome6). Symptoms of fat malabsorption such as steatorrhea or fatty food intolerance may suggest exocrine pancreatic dysfunction, which is a hallmark of Shwachman-Diamond syndrome. These symptoms often abate over time so the underlying diagnosis of Shwachman-Diamond syndrome is easily masked. Transient transaminitis with hepatomegaly and eczema in infancy are additional features of Shwachman-Diamond syndrome. Gastrointestinal symptoms such as diarrhea, vomiting, constipation, food intolerances, poor appetite, and abdominal pain are frequent features of Fanconi anemia or dyskeratosis congenita. Frequent unusual infections may be a sign of an underlying immune deficiency that may be associated with dyskeratosis congenita or Shwachman-Diamond syndrome. Pulmonary symptoms, often secondary to restrictive pulmonary disease or pulmonary vascular disease, are a common feature of dyskeratosis congenita. Cardiac anomalies have been described for Fanconi anemia, dyskeratosis congenita, Diamond-Blackfan anemia, and Shwachman-Diamond syndrome. Osteopenia has been described for many of the inherited marrow failure syndromes and may lead to pathologic fractures in a subset of patients. A wide range of endocrinopathies and hypogonadism have been associated with many of the inherited marrow failure syndromes. Symptoms of hypogonadism such as delayed puberty, amenorrhea, premature menopause, or infertility may be ascertained. Developmental delay or learning disabilities have been described in many of the IBMFS. Congenital amegakaryocytic thrombocytopenia is in the differential diagnosis of the neonate with bleeding and thrombocytopenia without any apparent cause such as infection, medications, maternal HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome, or antibody-mediated destruction. Idiopathic red cell aplasia presenting in the first year of life, particularly in association with red cell macrocytosis, is a common presentation of Diamond-Blackfan anemia.
As part of the evaluation of any patient with marrow failure, additional potential etiologies for secondary marrow failure should be sought in the history, including symptoms of infection, medications, autoimmune disease, malignancy, exposure to marrow toxins, or other marrow diseases.
Family History
Family history provides important clues to the diagnosis of an inherited marrow failure syndrome. A history suggestive of cancer predisposition, particularly cancers arising at an unusually young age without apparent etiology or risk factors, raises suspicion for an IBMFS. The spectrum of familial cancers may also be informative. For example, an autosomal dominant family history of oral, esophageal, GI, colorectal, or female genital tract cancers, acute myeloid leukemia (AML), or lymphomas may be seen with certain subtypes of dyskeratosis congenita.7 While Fanconi anemia shares a predisposition to many of these cancers, the inheritance pattern is recessive. A history of excessive toxicity from chemotherapy or radiation therapy, including prolonged severe pancytopenia or mucositis, may be associated with the IBMFS. Any family history of unexplained cytopenias or aplastic anemia should also be sought. Attention should be paid to non-hematologic manifestations of the inherited marrow failure syndromes, since many affected family members may fail to manifest significant marrow failure. For example, pulmonary fibrosis, liver fibrosis, or osteopenia are features of dyskeratosis congenita. A family history of unexplained fetal loss or congenital anomalies may also be associated with inherited marrow failure syndromes. Diagnostic testing is recommended for all siblings of patients with inherited marrow failure syndromes. Testing of parents is also helpful for syndromes with a dominant pattern of inheritance.
Physical Exam
A careful evaluation for physical signs of the inherited marrow failure syndrome may yield important clues to the underlying diagnosis. The physical findings associated with IBMFS are generally not pathognomonic in isolation, but warrant consideration in the context of concurrent marrow failure. Generally speaking, congenital anomalies in association with seemingly idiopathic cytopenias, even when mild, warrant consideration of a possible IBMFS. An important caveat, however, is that the conventional physical findings classically associated with these syndromes may be absent in affected patients. The triad of leukoplakia, rash, and nail dystrophy associated with dyskeratosis congenita is typically lacking in early life and may remain absent even into adulthood in a subset of patients.4 Unexplained short stature is a frequently overlooked feature of the IBMFS. Importantly, normal stature is insufficient to rule out these syndromes.
A list of all the physical findings associated with the IBMFS is beyond the scope of this review and can be found in standard textbooks and review articles. A few common features will be highlighted briefly here (Table 1 ).
Head
Facial features may provide important clues. For example, microphthalmia, hypotelorism, epicanthal folds, and a broad nasal bridge may be associated with Fanconi anemia. Cleft palate and micrognathia are associated with Diamond-Blackfan anemia.
Skeletal
Thumb anomalies and radial ray defects are frequent features of Fanconi anemia or Diamond-Blackfan anemia. Thumb anomalies range from severe deformities requiring surgical intervention to mild subtle abnormalities such as a hypoplastic thenar eminence or asymmetrical radial pulses. Some patients may also have anomalies of the toes. Skeletal anomalies, including thoracic dystrophies, have also been described for Shwachman-Diamond syndrome. A wide variety of skeletal abnormalities have been described for Fanconi anemia, including vertebral anomalies frequently attributed to VATER/VACTERL syndrome.
Skin
Skin pigmentary abnormalities such as hyper- or hypo-pigmentation are described with Fanconi anemia. A reticulated or mottled rash is associated with dyskeratosis congenita. An eczematous rash, usually in infancy, is a frequent feature of Shwachman-Diamond syndrome. Nail dystrophy or leukoplakia characteristic of dyskeratosis congenita is frequently mistaken for a fungal infection or thrush, respectively.
Cardiopulmonary
Cardiac structural anomalies are described for Fanconi anemia, dyskeratosis congenita, and Diamond-Blackfan anemia, while cardiac functional abnormalities have been associated with Shwachman-Diamond syndrome. Pulmonary disease, including pulmonary fibrosis or pulmonary vascular disease, may develop in patients with dyskeratosis congenita.
Genitourinary
Genitourinary abnormalities such as pelvic kidney, single kidney, horseshoe kidney, ureteral or urethral abnormalities are common features of several of these syndromes. Gonadal abnormalities such as hypogonadism, micropenis, and undescended testes are also common features.
Laboratory Evaluation
Hematology
Peripheral cytopenias may be severe, mild, or even absent. Unexplained red cell macrocytosis, particularly in the setting of a family history of marrow failure, cancer predisposition or physical anomalies, may be the sole hemato-logic abnormality. Other causes of low blood counts or red cell macrocytosis should be ruled out. Macrocytosis may be masked in patients with concurrent iron deficiency or thalassemia trait. Elevated hemoglobin F levels are commonly associated with IBMFS.
There are no pathognomonic findings on the bone marrow evaluation to diagnose the IBMFS. The marrow examination is helpful to rule out other disorders, such as a malignancy, to follow marrow cellularity, and to assess for cytogenetic clonal populations. Marrow cellularity must be interpreted within the context of global marrow function since cellularity is patchy and subject to sampling bias. The bone marrow morphology may exhibit dysplastic changes such as micromegakaryocytes with only single or double nuclei, pseudo–Pelger Huët anomalies, mild megaloblastic features with nuclear:cytoplasmic dissynchrony, or multi-nucleated erythroid precursors. The distinction between an IBMFS and myelodysplastic syndrome may be difficult based on morphologic features or cytogenetics alone. For patients with features suggestive of an IBMFS by history or exam, additional laboratory testing is recommended.
Chromosomal Breakage
The current gold standard for diagnosing patients with Fanconi anemia is the demonstration of increased chromosomal breakage following exposure to clastogens such as mitomycin C (MMC) or diepoxybutane (DEB). This response to MMC or DEB distinguishes Fanconi anemia from most of the other chromosomal breakage syndromes such as ataxia telangiectasia and Bloom’s syndrome. The exception is Nijmegen breakage syndrome, where increased chromosomal breakage may be difficult to distinguish from Fanconi anemia.8 Clinical findings and genetic testing are helpful in distinguishing Nijmegen breakage syndrome from Fanconi anemia.
Chromosomal breakage testing is typically performed on phytohemagglutinin (PHA)-stimulated peripheral blood lymphocytes. Reversion of the Fanconi anemia gene mutation in a somatic cell, typically a lymphocyte, may result in a falsely negative chromosomal breakage test,9,10 particularly given the relative growth advantage of the reverted clone. For patients with a high clinical suspicion for Fanconi anemia but a negative blood test, the diagnosis of Fanconi anemia may be made by testing skin fibroblasts for chromosomal breakage.
There are at least thirteen different Fanconi anemia subtypes identified at the time of this writing. Certain FA subtypes, such as FA-D111,12 and FA-N,13 are associated with increased risks of malignancies at a very young age and warrant closer monitoring. Identification of FA subtypes also allows for mutation identification, which is a prerequisite for preimplantation genetic diagnosis or carrier testing.
Telomere Length
Dyskeratosis congenita is caused by mutations in genes that affect telomere maintenance (see accompanying article by RT Calado, beginning on page 338). Telomeres are the specialized DNA:protein structures at the ends of chromosomes that prevent the genomic rearrangement of free DNA ends and allow replication of the distal chromosomal ends. Telomerase is the enzyme required for DNA replication at the telomeres. Telomere length shortens as a function of age. Although short telomeres are commonly seen in patients with marrow failure, telomere length is markedly shorter (<1st percentile) in patients with dyskeratosis congenita, even in comparison to other patients with marrow failure. Telomere length is emerging as a useful screen for dyskeratosis congenita, although experience with this test is still limited and interpretation must be made within the clinical context. Granulocytes in particular commonly exhibit shortened telomeres. A study measuring telomere lengths in a panel of five lymphocyte subsets plus granulocytes reported that telomere lengths below the 1st percentile for age in at least three different lymphocyte subsets strongly correlated with the diagnosis of dyskeratosis congenita.14 The interpretation of telomere length in relatives lacking any clinical findings of dyskeratosis congenita warrants further study.15 Genetic testing for mutations in dyskeratosis congenita genes is recommended to confirm the diagnosis if the telomere length analysis is suspicious for dyskeratosis congenita or if clinical suspicion for dyskeratosis congenita is high. Unfortunately, the absence of mutations in the DC genes identified to date is insufficient to rule out the diagnosis of dyskeratosis congenita, and it is likely that additional genes for dyskeratosis congenita remain to be identified.
Exocrine Pancreatic Testing
One of the hallmarks of SDS is exocrine pancreatic atresia but only a subset of patients manifest clinical symptoms. Only a small fraction (less than 2%–5%) of the exocrine pancreas is required to maintain adequate digestive capacity to be clinically silent. Steatorrhea resolves in half of patients with Shwachman-Diamond syndrome presenting with this symptom, rendering diagnosis elusive. Pancreatic stimulation testing is an invasive procedure so alternative tests are currently preferred. Measurement of serum trypsinogen and pancreatic isoamylase provides a useful screen for exocrine pancreatic dysfunction.16 Normal values for pancreatic isoamylase vary with age. Since pancreatic isoamylase levels are typically low before the age of 3 years even in healthy controls, this test is best utilized in older patients. On the other hand, serum trypsinogen levels often rise into the normal range beyond the age of 3 years even in patients with SDS, so this test is more sensitive in younger patients. The utility of the trypsinogen and pancreatic isoamylase testing is limited by the lack of clinical laboratories with clearly defined cut-off values distinguishing patients with Shwachman-Diamond syndrome from healthy controls at this time of this writing. An atretic fatty pancreas on imaging studies in a patient with marrow failure also suggests the diagnosis of SDS.17 Although fecal elastase has not been validated as a measure of exocrine pancreatic dysfunction in patients with Shwachman-Diamond syndrome, it is a widely used marker in cystic fibrosis, and a very low fecal elastase level would warrant further investigation.
Erythrocyte Adenosine Deaminase
Patients with Diamond-Blackfan anemia often have elevated erythrocyte adenosine deaminase (eADA) levels.18 The reason for this finding is unknown, but this has nonetheless proven a useful diagnostic marker. Elevated eADA and fetal hemoglobin levels, red cell macrocytosis, congenital anomalies, familial anemia, and age less than 1 year favors the diagnosis of Diamond-Blackfan anemia rather than transient erythroblastopenia of childhood (TEC). Laboratory testing must be interpreted cautiously during the recovery phase of TEC as some overlap with features of DBA may transiently arise.
Genetic testing
Many of the genes responsible for these syndromes have been elucidated (Table 2 ); however, negative genetic testing is insufficient to rule out these syndromes. Likely additional genes remain to be identified.
Fanconi Anemia
Fanconi anemia follows an autosomal recessive mode of inheritance with the exception of the FA-B subtype which is X-linked.3,19,20 The Fanconi anemia genes function coordinately to repair DNA damage, although the precise function of the FA proteins is still under investigation. Additional functions of the Fanconi anemia genes in stress responses, oxidative stress, and cytokine-mediated apoptosis have been described.3,21 The FANCD1 gene was previously identified as the tumor suppressor gene BRCA2. The FANCJ/BACH1/BRIP1 gene encodes a helicase, which is an enzyme that unwinds DNA. The FANCM (Hef) gene shares regions of homology with both helicases and endonucleases, though these functions have yet to be directly demonstrated. FANCM may function in DNA translocation. The FANCL/PHF9/POG gene shares homology with other E3 ubiquitin ligases. The Fanconi anemia proteins FANCA, FANCB, FANCC, FANCE, FANCF, FANCG, FANCL, and FANCM form a complex that activates the monoubiquitination of FANCD2 and FANCI following DNA damage. Ubiquitinated FANCD2 and FANCI in turn translocate to chromatin at sites of DNA damage where they associate with the FA proteins FANCD1/BRCA2, FANCN/PALB2, FANCJ/BRIP1/BACH1, as well as additional DNA repair proteins such as BRCA1, NBS, and RAD51 (Figure 1 ).
Dyskeratosis Congenita
There are three genetic modes of transmission for dyskeratosis congenita: X-linked, autosomal recessive, and autosomal dominant.4,22 The X-linked form is associated with mutations in the DKC1 gene, which encodes the dyskerin protein. The autosomal recessive form of dyskeratosis congenita is associated with mutations in NOP10/NOLA3 and NHP2/NOLA2. Dyskerin, NOP10, and NHP2 together with GAR1 form a core complex that binds to the H/ACA class of small nucleolar ribonucleoproteins (reviewed in Meier23) implicated in a variety of cellular functions including ribosome biogenesis through pseudouridylation, RNA splicing, and comprise components of the telomerase complex. The genes encoding the RNA component of telomerase, TERC, and the telomerase reverse transcriptase, TERT, are associated with the autosomal dominant form of dyskeratosis congenita. An additional gene, TINF2, encodes the TIN2 protein, a component of the shelterin complex that associates with the telomeres and is important for telomere maintenance.6 A significant proportion of dyskeratosis congenita patients lack any identifiable mutations in the known dyskeratosis congenita genes.
Of note, the dyskeratosis congenita genes comprising the H/ACA core complex have been implicated in additional cellular functions, particularly ribosomal RNA maturation. Dyskerin functions as an rRNA pseudouridylase. The pseudouridylated residues in the rRNA sequence are highly conserved and have been postulated to function in rRNA secondary structure or RNA:protein interactions.
Shwachman-Diamond Syndrome
Up to 90% of patients meeting clinical criteria for Shwachman-Diamond syndrome24 harbor biallelic mutations in the SBDS gene.25 The majority of mutations appear to arise from a gene conversion event with its adjacent pseudogene.25 The SBDS gene encodes a high conserved protein with a ubiquitous tissue expression pattern. The SBDS protein functions in ribosome biogenesis, possibly by promoting the joining of the 40S and 60S ribosomal subunits.24,26 SBDS also functions during mitosis by binding and stabilizing the mitotic spindle.27 SBDS has also been implicated in actin polymerization during neutrophil chemotaxis.28
Congenital Amegakaryocytic Thrombocytopenia
Congenital amegakaryocytic thrombocytopenia29 is an autosomal recessive disorder caused by mutations in the c-MPL gene.29 The c-MPL gene encodes the thrombopoietin receptor. Thrombopoietin is important for megakaryocyte differentiation as well as for the maintenance of hematopoietic stem cells. Type I patients have complete loss of c-MPL function and typically have a more severe presentation earlier in life. Type II patients maintain partial c-MPL function and may follow a milder clinical course.30
Diamond-Blackfan Anemia
Rare cases of severe aplastic anemia have been reported in patients with Diamond-Blackfan anemia.31 The list of genes responsible for DBA is rapidly growing, but to date all DBA genes encode protein components of either the small 40S or large 60S ribosomal subunits32,33 (see Table 2 ). DBA was the first IBMFS in which a ribosomal disorder was identified. Furthermore, acquired deletions of the gene encoding the RPS14 ribosomal protein have been identified in patients with 5q- MDS.34 Thus ribosomal pathways have been implicated in both marrow failure and MDS. The molecular mechanisms whereby insufficiency of ribosomal proteins lead to hematologic disease are under active investigation, but may involve activation of checkpoint pathways35,36 or translational regulation of specific mRNA subsets37 (see Lipton and Ellis32 for further discussion).
Summary
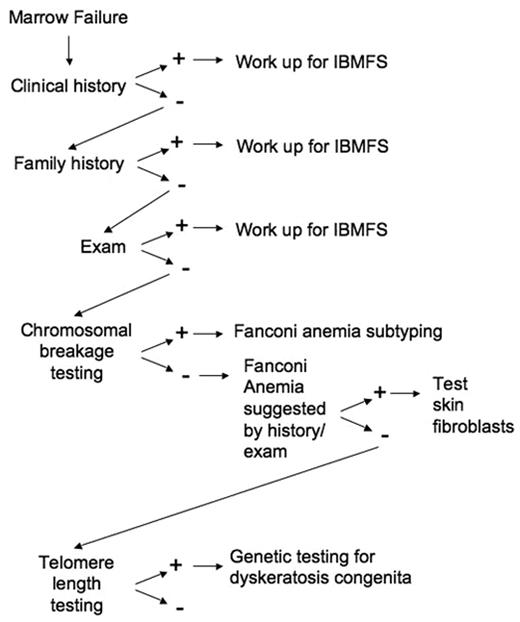
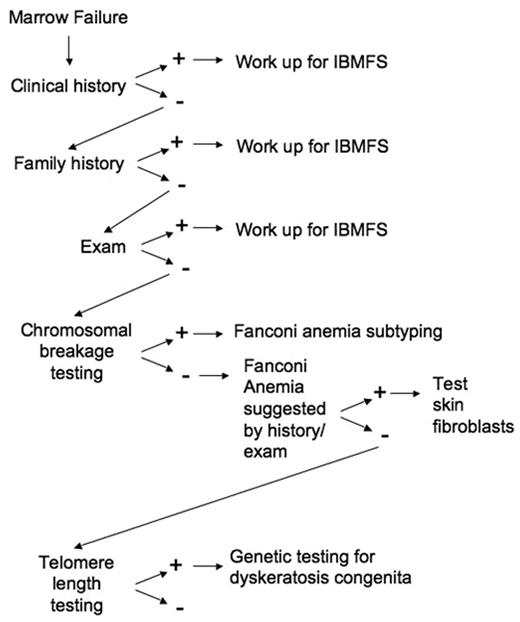
The early recognition of an inherited bone marrow failure syndrome informs clinical decision-making with regard to medical management and treatment. Early diagnosis also allows family counseling and appropriate donor selection for hematopoietic stem cell transplant. Careful clinical evaluation provides important clues to these often unrecognized syndromes (Figure 2 ). Insights into the molecular pathways underlying these rare disorders continue to elucidate global mechanisms contributing to marrow failure and malignant transformation in the general population.
Summary of molecular pathways in the inherited marrow failure syndromes.
Approach to the patient with marrow failure. A general outline of the evaluation of the patient with marrow failure is diagramed. See text for specific workup for each IBMFS as directed by history and exam.
Approach to the patient with marrow failure. A general outline of the evaluation of the patient with marrow failure is diagramed. See text for specific workup for each IBMFS as directed by history and exam.
Disclosures Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
Acknowledments
The author apologizes to all those whose work could not be cited in this review due to space limitations. Many of these citations may be found in the recent reviews referenced throughout this paper.
References
Author notes
Fred Hutchinson Cancer Research Center, Seattle, WA; Seattle Children’s Hospital, Seattle, WA