Abstract
The Wiskott-Aldrich syndrome (WAS) is an X-linked immunodeficiency disease with a characteristic clinical phenotype that includes thrombocytopenia with small platelets, eczema, recurrent infections due to immunodeficiency, and an increased incidence of autoimmune manifestations and malignancies. The identification of the molecular defect in the WAS gene has broadened the clinical spectrum of disease to include chronic or intermittent X-linked thrombocytopenia (XLT), a relatively mild form of WAS, and X-linked neutropenia (XLN) due to an arrest of myelopoiesis. The pathophysiological mechanisms relate to defective actin polymerization in hematopoietic cells as a result of deficient or dysregulated activity of the WAS protein (WASp). The severity of disease is variable and somewhat predictable from genotype. Treatment strategies therefore range from conservative through to early definitive intervention by using allogeneic hematopoietic stem cell transplantation and potentially somatic gene therapy. All aspects of the condition from clinical presentation to molecular pathology and basic cellular mechanisms have been reviewed recently.
Clinical Manifestations and Immunopathology
The incidence of the classic Wiskott-Aldrich syndrome (WAS) phenotype has been estimated to be between one and ten in one million individuals, although it is likely to be higher.1–3 Clinical manifestations suggesting WAS/X-linked thrombocytopenia (XLT) are often present at birth and consist of petechiae, bruising, and bloody diarrhea. Severe eczema is a frequent manifestation during infancy and childhood. The most consistent hallmark finding at diagnosis of both classic WAS and XLT is microthrombocytopenia. Infections, including purulent otitis media, pneumonia (most often caused by bacteria, rarely by Pneumocystis carinii), and skin infections, are common during the first 6 months of life. Patients with XLT have fewer problems with eczema and infections and are often misdiagnosed as having idiopathic thrombocytopenia (ITP). The severity of immunodeficiency may vary from family to family depending largely on the mutation and its effect on protein expression.4,5 Patients with XLT by definition have minimal immunological disturbances. During infancy, the number of circulating lymphocytes may be normal or moderately decreased.6 Later, lymphopenia due to reduced T-lymphocyte numbers is a common finding. The number of B cells may be normal or moderately depressed.7 Serum IgG levels are generally within normal range, IgM levels are usually depressed and IgA and IgE are frequently elevated. Antibody responses are adequate to some antigens and insufficient to others. Consistent findings are low isohemagluttinin titers and markedly depressed serological responses to polysaccharide antigens. Abnormal T-cell function is indicated by diminished proliferative responses particularly through TCR activation. WAS patients also have defective cytolytic, phagocytic and antigen presenting function.8–12
Thrombocytopenia associated with small platelet volume is an invariable finding in both classical WAS and XLT and is a key diagnostic indicator. Platelet counts may vary considerably, but are usually severely depressed. Intermittent thrombocytopenia has been described in two families with very mild disease.13 In most patients with WAS/XLT, the mean platelet volume is about half that of normal control subjects. After splenectomy, platelet counts and platelet volume usually increase substantially but may not normalize. This partial recovery of platelet counts following splenectomy suggests that thrombocytopenia in patients with WAS/XLT is at least in part due to platelet destruction in the spleen. As a consequence of the platelet defect, many WAS patients have a history of bleeding including intracranial hemorrhage.
Eczema is one of the characteristic findings that, in its most severe form, is resistant to therapy and persists into adulthood. Molluscum contagiosum, herpes simplex, or bacterial infections may develop in affected areas of the skin. XLT patients have either mild and transient eczema or none at all. Autoimmune diseases are frequent, and include most commonly hemolytic anemia followed by vasculitis, renal disease, Henoch-Schönlein–like purpura, and inflammatory bowel disease.14 Other less frequent autoimmune diseases include neutropenia, dermatomyositis, recurrent angio-edema, uveitis, and cerebral vasculitis. The incidence of autoimmune disease in XLT patients is generally less frequent than in classic WAS, but it can occur and indicates underlying immunological deregulation in even mild patients. Malignant hematological tumors can occur during childhood but are more frequent in adolescents and young adults with the classic WAS in whom immunodeficiency is profound. The most frequent malignancy reported is Epstein-Barr virus–related B-cell lymphoma. Some younger patients may also present with marrow dysplasia.
Lymph nodes and spleens from patients with WAS consistently show relative depletion of lymphocytes from T-cell areas. Depletion of the splenic (and circulating) marginal zone B cells is characteristic in patients with classical WAS and mouse models, and may explain the defective antibody responses, particularly to polysaccharide antigens.
Physiological Activity of WASp
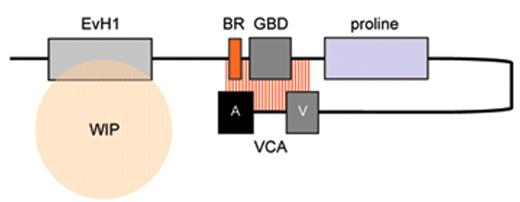
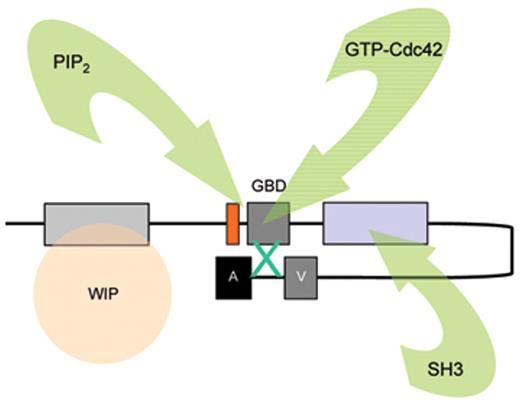
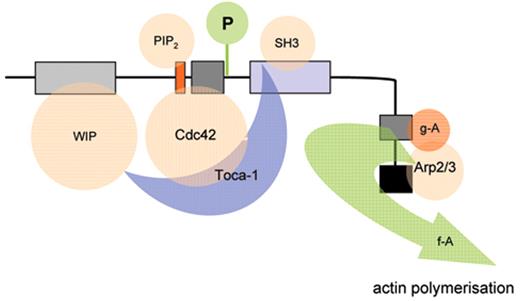
The biological mechanisms responsible for the pathophysiology of WAS and X-linked neutropenia (XLN) are directly linked to dysregulated actin polymerization in hematopoietic cells as a result of deficiency or constitutive overactivity of the WAS protein (WASp). WASp is a member of a distinct family of proteins that participate in the transduction of signals from the cell surface to the actin cytoskeleton. They are characterized by a C-terminal tripartite domain containing a common actin monomer-binding motif, WASp-homology domain 2 (WH2) or verprolin homology domain (V), and a central-acidic (CA) region which is capable of activating the actin related protein (Arp)2/3 complex, a potent nucleator of actin polymerization. The activities of WASp family members are normally tightly controlled within a cell, allowing for both spatial and temporal regulation of actin polymerization. WASp is regulated by several mechanisms including the adoption of an autoinhibited conformation in which the VCA domain forms a hydrophobic interaction with the GTPase binding domain (GBD, residues 230–288) and adjacent C-terminal residues (Figure 1 ). Binding of GTP-loaded Cdc42 and phosphatidylinositol 4,5-bisphosphate (PIP2) appear to cooperatively disrupt this interaction, thereby freeing the C-terminus for binding to the Arp2/3 complex. Serine and tyrosine phosphorylation (Y291 represented by “P” in Figure 1 ) of WASp also acts to directly regulate its activity, although until recently this has been relatively unexplored, particularly in vivo. The N-terminus of WASp/N-WASp contains an Ena/VASP homology 1 (EVH1), which binds the widely expressed verprolin homologue, WIP (WASp-interacting protein).15 The majority of WASp in cells is complexed with WIP, and the WIP/WASp interaction is important for WASp activation by Cdc42 through Toca-1. WIP is a molecular chaperone crucial for the stability of WASp, which otherwise is susceptible to proteolytic cleavage through calpain and possibly the 26S ubiquitin-proteasome systems.16–18 Very recently, an additional level of regulation has been proposed through dimerization of WASp at sites of actin polymerisation.19
Cellular Abnormalities Caused by Defective Actin Polymerization
The development of an organized immunological system for host defence and maintenance of tolerance is dependent on the ability of immune cells to respond to growth, differentiation, and localization signals. The effects of WAS gene mutations on these processes has therefore become of considerable interest, particularly as the actin cytoskeleton plays such a prominent role in the basic mechanisms of cell adhesion and migration.20–22 Migration through tissues and endothelial barriers is a complex series of events requiring a highly regulated cycle of cell protrusion, retraction, adhesion and detachment, and major dynamic rearrangements of the actin cytoskeleton. Myeloid and lymphoid cells from WAS patients and from WASp-deficient mice have been shown to be defective in their migratory and in vivo trafficking behavior in several ways. While there is no doubt that cell migration is compromised in the absence of WASp, the cell biological events that contribute to these defects have not been absolutely determined. It is possible that abnormalities of cell polarity and chemotaxis relate to a failure to produce normal protrusions such as filopodia. Similarly, efficient adhesion and detachment from surfaces is tightly linked to cell motility. WASp-deficient macrophages and immature dendritic cells (DCs) lack specialized adhesion structures known as podosomes.18 In these cell types, podosomes concentrate β2 integrins around an actin core and, although their function has not been clearly identified, are responsible for tight adhesion of cells to ICAM-1 and possibly JAM-A. Furthermore, they are highly dynamic with a turnover of minutes, providing a migrating cell with a mechanism for rapid attachment and detachment, and with localized anchorage points that could facilitate diapedesis. Interestingly, the sealing zone of bone-resorbing osteoclasts is also defined by circular clusters of podosomes that associate with αvβ3 integrins. The absence of podosomes in WASp-deficient murine osteoclasts has been shown to result in partially defective bone resorption, although the relevance to human disease has not been characterized.23 DCs are consequently compromised in their ability to migrate to secondary lymphoid organs, but also probably in their ability to form effective cognate interactions with T cells. T lymphocytes respond less well than normal cells in vitro to SDF-1 and CCL-19 and demonstrate abrogated homing to secondary lymphoid tissue following adoptive transfer in vivo.24–26 Interestingly, cells that are deficient for both WASp and WIP exhibit much more profound deficiencies than either alone, indicating that there is some redundancy.26 Similarly, WASp-deficient B lymphocytes have been shown to have marked morphological abnormalities, defective migration and adhesion in vitro, and impaired homing in vivo.27 This is likely to contribute to the observed deficiencies of humoral responses to both T-dependent and T-independent antigens, and to the marked deficiency of marginal zone B cells in both murine and human spleens.27–30
WASp-deficient T cells characteristically exhibit defective proliferation and actin rearrangement in response to CD3 co-receptor ligation. The fact that WASp is critical for cytoskeletal remodeling downstream of the T-cell receptor (TCR) is highlighted by its role in formation of the immune synapse (IS), which in the absence of WASp is partially defective.31–33 WASp-deficient CD4+ cells have in addition been shown to be unable to polarize cytokine secretion towards antigen-specific target cells, whereas chemokine secretion appears to be unaffected, indicating that the respective secretory pathways are distinct and differentially dependent on WASp.34 WASp is necessary for APC cytoskeletal remodeling during formation of the DC-natural killer cell (NK) immunostimulatory synapse and for subsequent DC induction of NK-cell interferon-γ production and killing.35 Similarly, impaired actin polymerization and perforin accumulation at the NK-target contact point has been shown to result in partially reduced NK cytolytic activity.8 Recently, the function of regulatory T cells and iNKT cells has been found to be compromised in both patients and mouse models.36–39 WASp appears to be necessary for integration of signals leading to nuclear translocation of NFAT2 and NF-κB (RelA) during cell-cell contact, which may be independent of its direct role in cytoskeletal rearrangement.40 The importance of WASp for normal B-cell signaling is less well established, although WASp deficiency results in abnormalities of morphology and impaired B-cell receptor capping.41–44
Molecular Pathology
Mutations of the WAS gene result in three distinct phenotypes: the classic WAS triad of thrombocytopenia/small platelets, recurrent infections, and eczema as first reported by Wiskott in 1937; the milder XLT variant,45,46 which can even be intermittent13; and congenital X-linked neutropenia (XLN) without any of the clinical finding characteristic of WAS/XLT (see below).45,47,48 In a cohort of 270 unrelated WAS/XLT families from single centers in the United States, Italy, and Japan, 158 unique WASP gene mutations were identified. The most common mutations observed were missense mutations (93 families) followed by splice site mutations (n = 59), short deletions (n = 46), and nonsense mutations (n = 39). Insertions, complex mutations, and large deletions were less frequent (12%). The amino acid substitutions were typically located in exons 1–4. Splice site mutations occurred predominantly in the downstream half of the WAS gene (introns 6–11). Mutational “hot spots,” defined as occurring in 7 or more unrelated families ( > 2.5%), were also identified in this cohort. Three hot spots represented point mutations within the coding regions and the other three involved splice sites. These six hotspot mutations account for 25.6% of the cohort of 270 families. Three of these mutations (168C>T;290 C>N/291 G>N and IVS6+5g>a) were consistently found in WASp-positive patients with a mild phenotype (XLT), whereas the three other mutations (665C>T, IVS8+1 g>n and IVS8+1 to +6 del gtga) were predominantly WASp-negative and had a high clinical score characteristic for WAS.
The most consistent phenotype-genotype correlation was observed when the patients were divided into two categories: WASp-positive if the mutated protein was expressed and of normal size and WASp-negative if the protein was absent or truncated.4,5 Patients with mutations that allowed expression of normal-sized mutated protein, often in reduced quantity, manifest, with few exceptions, the XLT phenotype, whereas those patients whose lymphocytes could not express WASp or expressed only truncated WASp were more likely to have the classic WAS phenotype. Progression to a score of 5 due to either autoimmune disease or malignancy was observed in both groups but was far more frequent in WASp-negative patients with an initial score of 3 to 4. There were, however, exceptions, making it difficult in individual cases to accurately predict the clinical course based solely on the type of genetic mutation.
Usually, molecular defects in the WAS gene result in diminished activity either because of aborted protein production, intrinsic instability of the mutant mRNA or protein, or because of disturbance to key regulatory interactions. For example, a significant proportion of WAS gene defects result in expression of mutant protein with amino acid substitutions within the EVH1 domain (exons 1–3). This would be predicted to disturb interaction with WIP to a variable extent depending on the precise molecular abnormality and therefore to dramatically compromise WASp stability.49 In contrast, three unique human mutations in the WAS gene have now been shown to cause very similar effects in terms of enhanced actin polymerization (L270P, S272P, I294T).48,50–52 Furthermore, all three missense mutations are located within the hydrophobic core and are predicted to prevent autoinhibition (perhaps in a similar way to physiological activation by phosphorylation of Y291). Interestingly, the phenotype of clinical disease arising from these mutations affecting the Cdc42 binding site is quite unlike that of classical WAS, and is characterized by neutropenia and monocytopenia as a result of inhibited myelopoiesis.51
A number of recent studies have demonstrated somatic mosaicism resulting from spontaneous reversion of an inherited disease-causing genetic mutation. Reversion has been detected molecularly in T and B and NK lymphocytes, although only revertant T cells have been detected in significant numbers peripherally.53 Whether this results in alleviation of clinical severity is open to question, and probably depends on the diversity and phenotype of revertant cells in individual patients.
Management of WAS/XLT Patients
Management of patients with WAS continues to present major challenges, particularly in attenuated phenotypes where the natural history of disease progression is less predictable (for overview see Burns and Thrasher54). A useful guide is provided by a scoring system as shown in Table 1 , although patients may progress in severity score with age (taken from Ochs and Thrasher, 2006).55 Although WASp-negative patients previously would not usually survive into adulthood without definitive treatment, current protocols undoubtedly have improved prognosis for this group of patients. Early diagnosis is most important for effective prophylaxis and treatment. Infants with classical WAS should receive Pneumocystis carinii pneumonia prophylaxis. As antibody responses to protein or polysaccharide antigens are defective, prophylactic immunoglobulin infusions at full therapeutic dose are recommended for all patients with classical WAS even though total IgG levels may be normal. Subcutaneous delivery is particularly convenient even with low platelet counts. Continuous additional antibiotic therapy should be considered if infections occur despite these measures. Live virus vaccines are not recommended although WAS patients do generally appear to mount effective protect serological responses to this type of challenge, particularly early on in the disease course when immunological attrition is less pronounced. Disseminated viral infections, particularly with herpes viruses, can be very severe even in infancy and require aggressive treatment. Complicated and extensive molluscum responds well to topical cidofovir. Eczema, if severe, requires aggressive therapy including topical steroids and if necessary short-term systemic steroids. Autoimmune manifestations may require more aggressive immunosuppression and may be refractory to conventional modalities. Many autoimmune phenomena (particularly blood dyscrasias) appear to be driven by autoreactive B-cell clones and may respond to monoclonal antibodies targeting the CD20 antigen (rituximab). This is a relatively safe therapy particularly as these patients will be receiving immunoglobulin replacement. Platelet transfusions are reserved to treat active bleeding, for example, central nervous system hemorrhages or gastrointestinal bleeding. Most patients do not develop severe hemorrhage except in situations of trauma or when thrombocytopenia is aggravated by autoimmunity. Blood products are best irradiated and should be cytomegalovirus-negative and where possible HLA-matched. Splenectomy is usually effective in increasing platelet numbers unless there is coincident autoimmunity, but the usual cautions apply with regard to pre-vaccination and lifelong antibiotic prophylaxis, particularly in XLT patients for whom splenectomy may be considered to enhance quality of life. At present, the only curative therapy for WAS is hematopoietic stem cell transplantation, with good results for patients with HLA-matched family or unrelated donors, or partially matched cord blood donors, but less satisfactory outcomes for other donor types.56,57 Autologous gene therapy is an emerging new technology and trials are ongoing58. At present, definitive treatment is usually not considered for XLT patients because of associated risks.
Model depicting mechanisms for activation of WASp. WASp is a multimodular protein, including an N-terminal EvH1 domain, a short basic region (BR) adjacent to a GTPase binding domain (GBD), a polyproline domain, and a tripartite C-terminal VCA domain (see main text for details). The majority of cytosolic WASp is complexed with WIP, which binds to the EvH1 domain. In the inactive state, WASp adopts an autoinhibited configuration in which the C region of the VCA module interacts with the GBD and adjacent sequences. In this configuration, either Arp2/3 complex cannot be bound or is inactive. WIP appears to play an important role in maintaining this configuration in the absence of activating signals. Activation following cell stimulation is initiated by several factors including GTP-bound CdC42, PIP2, and many SH3 domain containing proteins that in some cases are responsible for phosphorylation of Y291. These individual factors may operate cooperatively, but may also be redundant for activation depending on the context. Destabilization of the autoinhibited conformation of WASp permits the initiation of actin polymerization for monomeric g-actin by the Arp2/3 complex. Toca-1 is essential for Cdc42 and PIP2-induced actin polymerization, and binds to both Cdc42 and a WIP-WASp complex through the SH3 domain of Toca-1. It is not clear whether Toca-1 can interact directly with WIP, or whether the WIP-WASp complex dissociates after binding.
Model depicting mechanisms for activation of WASp. WASp is a multimodular protein, including an N-terminal EvH1 domain, a short basic region (BR) adjacent to a GTPase binding domain (GBD), a polyproline domain, and a tripartite C-terminal VCA domain (see main text for details). The majority of cytosolic WASp is complexed with WIP, which binds to the EvH1 domain. In the inactive state, WASp adopts an autoinhibited configuration in which the C region of the VCA module interacts with the GBD and adjacent sequences. In this configuration, either Arp2/3 complex cannot be bound or is inactive. WIP appears to play an important role in maintaining this configuration in the absence of activating signals. Activation following cell stimulation is initiated by several factors including GTP-bound CdC42, PIP2, and many SH3 domain containing proteins that in some cases are responsible for phosphorylation of Y291. These individual factors may operate cooperatively, but may also be redundant for activation depending on the context. Destabilization of the autoinhibited conformation of WASp permits the initiation of actin polymerization for monomeric g-actin by the Arp2/3 complex. Toca-1 is essential for Cdc42 and PIP2-induced actin polymerization, and binds to both Cdc42 and a WIP-WASp complex through the SH3 domain of Toca-1. It is not clear whether Toca-1 can interact directly with WIP, or whether the WIP-WASp complex dissociates after binding.
Disclosures Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
References
Author notes
Institute of Child Health, London, United Kingdom