Abstract
Acute promyelocytic leukemia (APL) was initially described as a distinct clinical entity in 1957, one year before the first meeting of the American Society of Hematology. With routine administration of all-trans retinoic acid (ATRA) combined with chemotherapy in the early 1990s and arsenic trioxide (ATO) in the late 1990s, cure can now be expected in the majority of both newly diagnosed and relapsed patients. ATRA with anthracycline-based chemotherapy for induction and consolidation followed by ATRA plus low-dose chemotherapy maintenance is currently the standard of care for newly diagnosed patients. Early institution of ATRA before the diagnosis is confirmed by genetics and aggressive blood product support are important to reduce induction mortality, which remains approximately 10% among patients entered on clinical trials, but is certainly higher when all patients are considered. The relapse rate among high-risk patients is approximately 20%, and new strategies include administration of intensified anthracyclines, intermediate- or high-dose ara-C in either induction or consolidation, or ATO as early consolidation. Central nervous system (CNS) prophylaxis for such patients and those with relapsed disease may be important to prevent the development of extramedullary disease. New therapeutic strategies have focused on minimizing chemotherapy and administering ATRA plus ATO as initial therapy. Recent studies suggest that patients who are molecularly negative after intensive consolidation may not benefit from maintenance therapy. Most exciting is the combination of ATRA and ATO, given with minimal chemotherapy only for leukocytosis, which is a very effective new strategy for patients who are unable to tolerate anthracyclines or older adults and soon may replace conventional therapy for many patients. Patients with relapsed disease do well with ATO followed by autologous hematopoietic stem cell transplantation
Introduction
Acute promyelocytic leukemia (APL) was first described by Hillestad as a distinct clinical entity 50 years ago.1 This was approximately 100 years after the earliest reports of several patients with enlargement of the spleen and peculiar appearance of the blood by Bennett and Virchow in 1845, who are credited with the initial descriptions of leukemia.2,3 More than 100 years passed after leukemia was described before the effective induction regimen for acute myeloid leukemia (AML) that includes an anthracycline and ara-C was identified, which has remained the standard of care for more than 30 years.4 However, during the 50 years since Hillestad’s report in 1957, rapid and unprecedented progress has been made in our understanding of the biology and treatment of APL. The molecular pathogenesis of APL has been deciphered,5 the natural history has been described, and treatment strategies have evolved such that the disease is now highly curable.6
Historically, this uncommon subtype of AML, accounting for 10% to 15% of approximately 13,400 new cases of adults with AML per year in the United States, was among the most fatal at presentation or during induction. This was primarily because of an associated complex and often catastrophic bleeding disorder which frequently resulted in intracerebral hemorrhage.7 Once a patient survived induction, the overall outcome was better than that of most other subtypes of AML with disease-free survivals of 40% to 50%. However, since the introduction of all-trans retinoic acid (ATRA) in the late 1980s administered together with anthracycline-based chemotherapy and arsenic trioxide (ATO) in the late 1990s, cure can be expected in the majority of both newly diagnosed and relapsed patients.6 However, early death is still frequently due to hemorrhage and remains a formidable problem.
Certain biologic and clinical features distinguish APL from other subtypes of AML and several contribute to the high cure rate. These include unusual sensitivity of the leukemia cells to anthracyclines, the presence of the PML-RARα fusion transcript, which results from the unique t(15;17) translocation,8,9 the capacity of the leukemia cells to undergo differentiation with ATRA, which targets the RARα moiety of the fusion transcript,10 and apoptosis with ATO, which targets the PML moiety.11 In addition, the leukemic stem cell in APL may arise in a more committed cell (a leukemic stem cell) than the hematopoietic stem cell from which other AMLs may derive.12 Therapeutic strategies continue to evolve rapidly, particularly in light of apparent synergism between ATRA and ATO. The five most important issues in the treatment of APL in 2008 will be addressed. These include the role of ara-C, the emerging place of ATO, the early death rate, optimal treatment for high-risk patients, and the benefit of maintenance.
Clinical Trials of ATRA in Patients with Newly Diagnosed APL
ATRA as a single agent has the remarkable ability to induce complete remission (CR) in most patients with relapsed or refractory APL.13,14 However, remissions are not sustained. Consolidation chemotherapy administered after induction with ATRA to newly diagnosed patients improves the remission duration.15 Cooperative groups carried out clinical trials to compare ATRA with conventional chemotherapy that included daunorubicin and ara-C, and to determine the optimal sequence of ATRA and chemotherapy.16,17 Such studies contributed to curative therapeutic strategies for newly diagnosed patients that became a new standard of care.
In the first North American Intergroup study (protocol I0129) ATRA was compared to conventional chemotherapy including daunorubicin and ara-C for induction.16 Patients in CR completed 2 courses of intensive consolidation chemotherapy and then were randomized to either maintenance with 1 year daily oral ATRA or to observation without maintenance. Patients who received ATRA for both induction and maintenance had the best outcome. Approximately 75% of patients appear cured of their disease. This study also demonstrated benefit to 1 year of daily oral ATRA as maintenance therapy. The European APL group conducted a randomized trial comparing sequential versus concurrent ATRA and chemotherapy for induction and showed a benefit to the concurrent approach.17 The Spanish cooperative group Programa de Estudio y Tratamiento de las Hemopatias Malignas (PETHEMA) took advantage of the peculiar sensitivity of leukemic promyelocytes to anthracyclines and carried out Phase II studies omitting ara-C from induction and consolidation.18,19 This strategy was supported by Bernard’s initial study7 and by a prospective randomized trial conducted by the Italian cooperative group Gruppo Italiano Malatte Ematologiche dell’Adulto (GIMEMA) in the pre-ATRA era showing no advantage to the addition of ara-C to idarubicin in patients with newly diagnosed APL.20 The disease-free survival (DFS) without ara-C varied between 81% for patients receiving standard doses of idarubicin and 90% when intermediate- and high-risk patients were given higher doses of idarubicin (risk-adapted therapy, discussed below).
Such studies established ATRA combined with anthracycline-based chemotherapy for induction, followed by anthracycline-based chemotherapy for consolidation and then maintenance as the standard of care.6 Long-term follow-up studies have confirmed the benefits of ATRA plus chemotherapy.21,22 The majority of patients appear to be cured with ATRA and anthracycline-based therapy. However, recent clinical trials report that approximately 10% die early. Clearly this is an underestimation of the overall early death rate in APL, since some patients die of hemorrhage before confirmation of diagnosis and before clinical trial registration. In addition, approximately 20% to 30% of patients relapse. Therefore, the identification of prognostic factors that can identify such patients has become a major focus of research.
Prognostic Factors in APL
Prognostic factors predicting for early death
The most important factor predicting outcome in patients with APL is the white blood cell (WBC) count at initial presentation. Prognostic factors that predict for early death due to hemorrhage include abnormal creatinine, increased peripheral blast count and presence of coagulopathy.23 In a retrospective analysis, Dally and colleagues found that severe bleeding did not correlate with WBC count at presentation.24 Older age is another important unfavorable prognostic factor, particularly for death in CR. It has been suggested that a reduction in the intensity of consolidation in older adults may be beneficial.25
Predicting relapse
The PETHEMA and the GIMEMA have used both initial WBC count and platelet count to classify patients into low, intermediate and high risk for relapse.24,26 Low-risk patients had a relapse-free survival (RFS) of 100% at 5 years whereas intermediate-risk patients had a RFS of 90% and high-risk patients had a RFS of 75%. This risk classification serves to identify patients at relatively high-risk of relapse, but also those at relatively low risk of relapse for whom minimizing or eliminating chemotherapy has become an important goal.
Other prognostic factors with no influence on therapeutic strategy
The presence of FLT3 internal tandem duplication mutations, seen in approximately 35% of APL patients and often associated with hyperleukocytosis, may confer an unfavorable prognosis with respect to induction mortality; however, the impact on relapse rate and overall survival (OS) is less clear.27,28 The presence of the bcr3 isoform of the PML-RARα fusion transcript may confer an unfavorable prognosis.29 However, there is no evidence that current therapy should be modified based on potential prognostic factors, such as cytogenetic abnormalities in addition to the t(15;17), CD56 expression, the presence of the FLT3 mutation, or the type of PML isoform. At the present time, current therapy should only be modified based on the presenting WBC, age and risk group classification. The identification of a risk classification system has paved the way for the development of risk-adapted therapeutic approaches.
The Role of Ara-C and New Strategies in Induction Therapy
The combination of ATRA plus idarubicin appears to be equally effective in inducing prolonged CR and likely cure as occurs when anthracyclines are combined with ara-C and ATRA.19,30 However, the European APL group carried out a prospective randomized trial comparing ATRA and daunorubicin to ATRA with daunorubicin and ara-C in patients younger than age 60 and with a WBC count of <10,000/μL.31 Although the CR rates were similar, 94% and 99% (P =.12) of patients treated with or without ara-C, respectively, the trial was discontinued early because of an increase in the relapse rate among patients treated without ara-C. The cumulative incidence of relapse at 2 years was 15.9% and 4.7%, respectively (P = .011). The EFS and OS rates were 77.2% and 93.3% and 89.6% and 97.9% (P = 0066), respectively. These data are difficult to interpret and compare to the results observed without ara-C reported by the PETHEMA because it is not known if the total cumulative doses of the two anthracyclines is similar, and patients in the PETHEMA studies received ATRA during consolidation. The Medical Research Council (MRC) in the United Kingdom randomized newly diagnosed patients under age 60 to ATRA plus 4 courses of standard chemotherapy including ara-C or to the approach reported by the PETHEMA of ATRA plus idarubicin without ara-C for 2 courses followed by 2 courses of mitoxantrone plus ATRA then 18 months of maintenance, but without any ara-C.32 Patients in both arms were randomized in course 3 to receive or not receive gemtuzumab ozogamicin. No difference in CR rate (91% vs 93%) was observed. After a median follow-up of 24 months, OS at 4 years was not different (81% MRC, 85% PETHEMA) and less myelosuppression was associated with the regimen omitting ara-C. It is possible that idarubicin is more effective than daunorubicin in APL. Alternatively, the cumulative doses of idarubicin administered may provide more anthracycline than doses of daunorubicin delivered in the European APL and MRC trials. However, it is clear that most patients with APL can be cured without ara-C and with potentially less toxicity.
The Emerging Role of Arsenic Trioxide
Arsenic trioxide in relapsed and refractory APL
Excellent results are obtained with ATO as a single agent in patients with relapsed and refractory APL.33,34 In the US multicenter trial of 40 patients previously treated to ATRA, approximately 50% of patients achieved complete molecular remission after induction with one 5-week course of ATO.34 Furthermore, approximately 86% of patients achieved complete molecular remission after 2 courses of ATO. A retrospective study from France compared ATO followed by chemotherapy with or without either autologous or allogeneic hematopoietic stem cell transplantation (HSCT) to ATRA plus chemotherapy with or without HSCT for patients with relapsed APL.35 Patients treated with the ATO-based regimen had an overall survival (OS) probability of 77% at 3 years compared to 47% for the patients treated with the ATRA plus chemotherapy-based regimen. The U.S. Multicenter trial and others have established ATO as the single most active agent in APL and have prompted studies which include ATO in induction.
Arsenic trioxide plus ATRA in induction
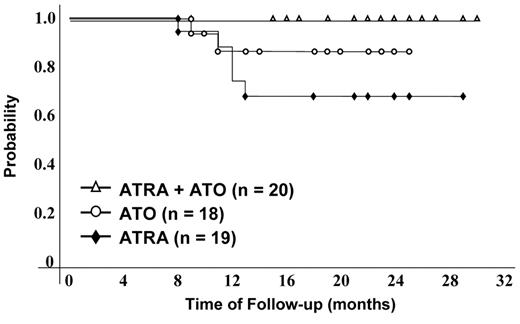
A prospective randomized trial in newly diagnosed patients with APL was conducted by investigators at the Shanghai Institute of Hematology in which patients were randomized to receive either ATRA, ATO or the combination of ATRA plus ATO (Table 1 ).36,37 All patients subsequently received intensive consolidation chemotherapy and all patients received maintenance therapy with the same agents used in induction. Patients randomized to the combination arm achieved complete hematologic and cytogenetic remission in a shorter time period (25 ± 5 days) compared to the time required among patients receiving either ATRA alone (40 ± 10 days) ATO alone (35 ± 3 days). Furthermore, there was a significantly greater reduction in the amount of PML-RARα fusion transcript among patients treated with the combination of ATRA and ATO compared with that with either agent alone. After follow-up of 18 months, among patients randomized to the combination therapy, there were no relapses compared to those randomized to either ATRA or ATO alone36 (Figure 1 ). In a follow-up report, among 60 patients treated with ATO plus ATRA for induction, the CR rate was 93.3% with a median time to CR of 27 days.37 With a follow-up of 48 months (range 25–60), the 4-year OS and event-free survival (EFS) were 98% and 94%, respectively. Fifty-six historical control patients were treated between 1998 and 2001 with ATRA until CR followed by chemotherapy and ATO only at relapse. With a median follow-up of 56 months, the 4-year OS and EFS were 83% and 46%, respectively.37 Investigators at the MD Anderson Cancer Center treated 44 newly diagnosed patients with ATRA and ATO for induction and consolidation and added either gemtuzumab ozogamicin or idarubicin in 19 high-risk patients to prevent hyperleukocytosis and/or the APL differentiation syndrome.38 The CR rate was 89% for all patients and 79% for those patients with high-risk disease. The RFS and OS were 90%. Although, this was not a prospective randomized trial, these data suggest a major benefit for the combination at ATO and ATRA with minimal chemotherapy in the treatment of APL.
Arsenic trioxide as a single agent for induction
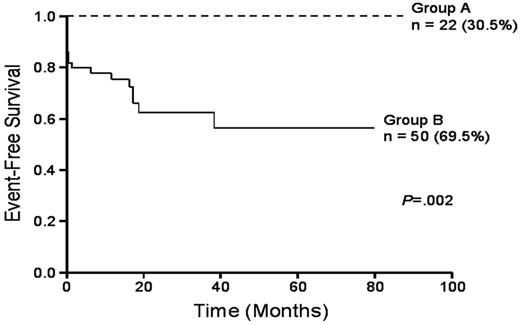
Single-agent ATO is very effective therapy for patients with newly diagnosed APL (Table 1 ).39–41 Investigators in Iran have treated patients with only 2 courses of ATO and have demonstrated an 86% CR rate and a 92% complete molecular remission rate although the relapse rate was relatively high at 26.3% at a median of 17 months.39 The 2-year DFS was 63.7%. Among 48 patients followed during the first year after CR, RT-PCR (sensitivity 10–3) for the PML-RARα fusion transcript was positive in only 4 patients (8.3%), of whom 3 relapsed. Investigators from India reported an identical CR rate among 72 patients given ATO for induction followed by up to 6 courses of ATO for consolidation.40 In this study, the CR rate, 3-year EFS, DFS and OS were 80%, 75%, 87% and 86% respectively. The complete molecular remission rate was 76%. Patients with a WBC < 5 × 109/L and a platelet count > 20 × 109/L fared particularly well with a 3-year EFS, DFS, and OS all of 100% compared to the outcome of other patients with a 3-year EFS, OFS, and OS of 63%, 80% and 79%, respectively (Figure 2 ). The outcome of other patients was not as favorable primarily because of a high early death rate attributable in part, according to authors, to inadequate supportive care. A small trial in children with newly diagnosed APL treated with single-agent ATO for 8 cycles over 12 months was reported by George and coworkers.41 Among 11 children treated, the CR rate was 91% and all 10 patients achieving CR achieved a complete molecular remission at a median of 81 days. The RFS was 81% and the OS 91%. While one small but randomized study has suggested that the addition of ATRA to ATO in patients with relapsed and refractory APL does not improve the outcome compared to ATO alone,42 in untreated APL the benefits of both agents given together may be important. The combination appears to induce a faster CR rate and a more profound complete molecular remission rate with no apparent greater toxicity than each agent alone.36 Therefore, among patients who cannot be treated with anthracyclines such as older adults or those patients who have abnormal cardiac function, or patients with low-risk or intermediate-risk APL may be treated cautiously with a combination of ATO plus ATRA for multiple cycles, preferably in the context of a clinical trial (Table 2 ). Large trials are currently underway. For most patients, 2 cycles of ATO alone do not appear sufficient to prevent relapse.39
Reducing Early Death in APL
Reducing early death in APL remains a formidable challenge. The rate of death during induction reported in clinical trials does not represent the actual early death rate among all patients with the disease. Although both ATRA and ATO ameliorate the coagulopathy, such improvement takes time and some patients present with intracerebral hemorrhage or have fatal bleeding before therapy can be initiated. The pathogenesis of coagulopathy is complex and is a manifestation of disseminated intravascular coagulation, primary fibrinolysis and direct proteolysis.43 ATRA should be initiated at the earliest suspicion of the disease without waiting for cytogenetic or molecular confirmation. Aggressive blood product support to maintain the platelet count ≥ 30,000 to 50,000/μL and the fibrinogen level at ≥ 150 mg/dL has been recommended.44 The role of recombinant activated factor VIIa remains to be established.45
New Strategies in Consolidation Therapy Focusing on High-Risk Patients
Increased Doses of Anthracyclines
The treatment of patients with high-risk APL is a major focus of research. Despite contemporary strategies, the 3-year cumulative incidence of relapse is approximately 20% to 30%.18,19 The addition of ATRA in consolidation, in patients with intermediate- and high-risk disease may improve outcome19 (Table 3 ). Patients on the LPA99 study received ATRA at the standard dose of 45 mg/m2 per day on days 1 through 15 in combination with the 3 single-agent chemotherapy courses of consolidation. The impact of adding ATRA in consolidation is somewhat confounded because patients in these risk groups also received an increased dose of idarubicin in the first course and received 2 consecutive days of idarubicin in the third course instead of 1 as given in a previous study (LPA96). Nevertheless, the relapse rate was lower and the survival rate was better among intermediate- and high-risk patients with ATRA in consolidation and higher doses of idarubicin, although these maneuvers influenced patients with high-risk disease the least.
Intermediate- or high-dose ara-C in consolidation
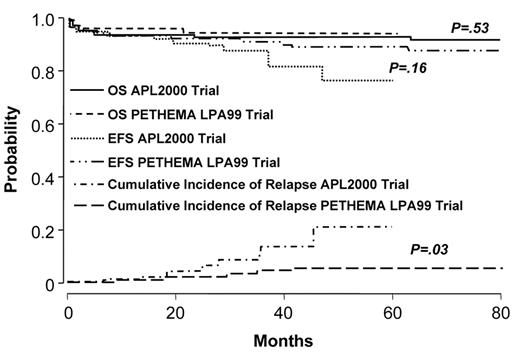
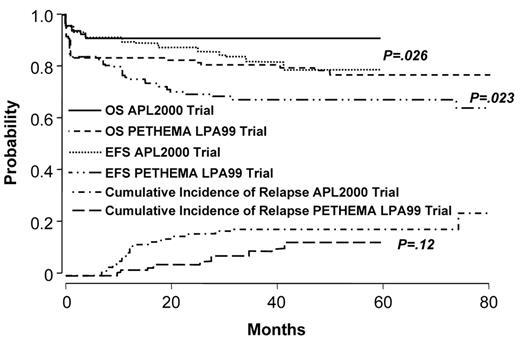
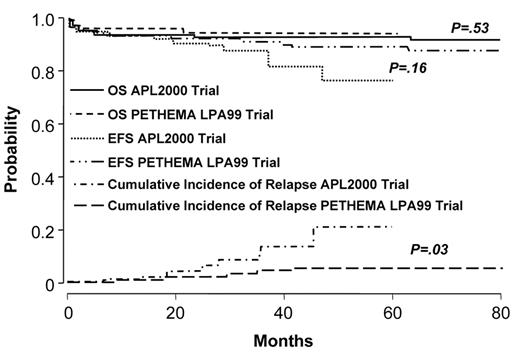
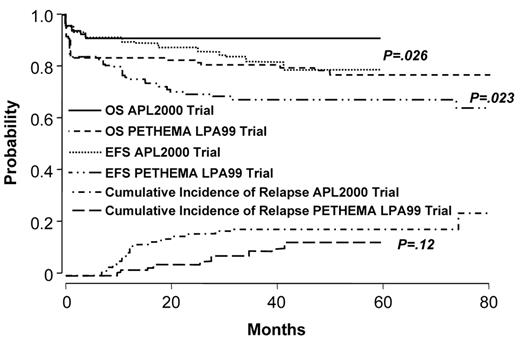
Two studies demonstrate that the administration of intermediate- or high-dose ara-C to patients at high-risk improves outcome.46,47 The German AML Cooperative Group (GAMLCG) administered high-dose ara-C (3 g/m2 dose) in induction and found that high WBC count was not a risk for relapse.46 Similarly, the GIMEMA cooperative group administered intermediate-dose ara-C during consolidation to patients with high-risk disease.47 In addition, such patients received ATRA in conventional doses for 15 days in consolidation. The first course of consolidation included ara-C in the dose of 1 g/m2 per day for 4 days together with idarubicin, the second course included mitoxantrone and etoposide and the third course included idarubicin, ara-C at 150 mg/m2 every 8 hours subcutaneously each day for 5 days and 6-thioguanine. The administration of intermediate- or high-dose ara-C has the advantage of providing levels of drug which cross the blood brain barrier. This fact may be important since patients with high-risk disease may be at increased risk for extramedullary disease.48–50 A recent comparison of outcome among high-risk patients treated with increased doses of anthracyclines or high-dose ara-C suggests that the latter is better51 (Figures 3aF3A and 3bF3B ). Patients with a WBC count ≥ 10,000/μL treated with high-dose ara-C had a higher CR rate (95.1% vs 83.6%, P = .018), a lower relapse rate at 3 years (9.9% vs 18.5%, P = .12) and a better OS (91.5% vs 80.8%, P = .026) than high-risk patients treated with increased doses of anthracyclines.
Arsenic trioxide in consolidation
In the North American Intergroup protocol C9710, after induction with ATRA, daunorubicin and ara-C in conventional doses, patients were randomized to receive or not receive two 25-day courses of ATO as a first consolidation.29,52 Patients subsequently received further consolidation with two courses of 3 days of daunorubicin with one week of ATRA. Patients receiving ATO had an improved EFS and OS compared to patients not receiving ATO. All risk groups appeared to benefit. However, patients not receiving ATO fared less well than patients treated similarly reported by other groups. Although the administration of ATO with the current recommended schedule of 25-day cycles is inconvenient, some patients may tolerate ATO better than intermediate- or high-dose ara-C. These data indicate that several strategies are effective for patients with high-risk disease. (Table 3 ) Such strategies are effective at preventing relapse in high-risk patients once they achieve CR.
Minimizing or Eliminating Chemotherapy in APL and New Concepts in Maintenance Therapy
Randomized trials in patients who are molecularly negative after consolidation
With current treatment strategies, patients with low-risk and intermediate-risk disease are almost always molecularly negative and have a 3–5 year disease-free survival (DFS) of approximately 80–85%.19 Although initial randomized trials suggested maintenance therapy was beneficial, recent studies indicate that such a strategy may not be helpful for patients who are in complete molecular remission after intensive consolidation.53,54 In a trial carried out by the GIMEMA between 1993 and 2000, 318 patients were randomized to one of four maintenance arms including oral 6-mercaptopurine (6-MP) and methotrexate, ATRA alone, ATRA alternating with 6-MP and methotrexate or no further therapy after consolidation.53 Beginning in 1998, 268 patients in complete molecular remission after consolidation were randomized to either ATRA alone or ATRA plus 6-MP and methotrexate for 2 years. A total of 78 PML-RAR alpha negative patients were randomized to 6-MP plus methotrexate, 83 to ATRA alone, 81 to ATRA plus 6-MP and methotrexate, and 76 to no further therapy. No difference in molecular DFS was observed among the randomized arms. In a second study carried out by the Japan Adult Leukemia Study Group (JALSG) (APL97) patients in complete molecular remission were randomized to either 6 courses of intensive maintenance chemotherapy or observation after consolidation, also found no benefit to maintenance.54 These studies suggest that patients who have low-risk or intermediate-risk disease, and who achieve complete molecular remission after consolidation therapy, do not benefit from maintenance. This issue is important because recent studies suggest a small, but potentially important incidence of secondary myelodysplastic syndromes and AML among patients treated with contemporary strategies including maintenance.55,56 Given the excellent outcome of patients receiving a combination of ATRA plus ATO or ATO alone among very low-risk patients (WBC ≥5,000/μL and platelets > 20,000/μL), it may be possible to eliminate not only maintenance therapy, but also induction and consolidation chemotherapy except in those patients who develop leukocytosis or the differentiation syndrome. A new North American Intergroup trial for patients with low-risk and intermediate-risk APL will randomize patients in complete molecular remission after induction and consolidation to receive or not receive maintenance therapy (S0521).
Curative Strategies in APL
Treatment of newly-diagnosed patients
The majority of patients with newly diagnosed APL should receive ATRA plus anthracycline-based chemotherapy for induction and consolidation (Table 4 ). Compelling data show that ATRA plus idarubicin alone is sufficient for the majority of low-risk and intermediate-risk patients. Alternatively, one can administer ATRA plus daunorubicin and ara-C. The major component of consolidation should also include anthracyclines, and ATRA may be beneficial. In general, patients should receive 2 to 3 cycles until the achievement in complete molecular remission. Patients with high-risk disease appear to have a lower risk of relapse if intermediate- or high-dose ara-C is given either in induction or consolidation (Figure 3aF3A and 3bF3B ). The latter may be easier to administer in consolidation when there is no coagulopathy present. ATO as an early consolidation also reduces the relapse rate and does so in all risk groups. Until further data are available, patients should receive ATRA with or without low-dose chemotherapy for 1 to 2 years for maintenance, acknowledging that maintenance therapy may not benefit patients who are in complete molecular remission after intensive consolidation. Monitoring of the peripheral blood by RT-PCR every 3 to 6 months for 2 to 3 years has been recommended. However, it is possible that such frequent monitoring may only be useful for those patients with high-risk disease. For patients who are not suitable candidates for anthracycline-based chemotherapy, such as those with significant cardiac disease or older adults, the combination of ATO plus ATRA is particularly effective. The introduction of oral arsenic derivatives in the near future will likely represent another important advance.57,58
Treatment of relapsed APL
For the few patients who relapse, ATO is the treatment of choice. The majority of patients will achieve complete molecular remission after two 25-day cycles.33 This is generally followed by the collection of peripheral blood autologous stem cells after a complete molecular remission has been achieved followed by high-dose chemotherapy and autologous hematopoietic stem cell transplantation. One can consider prophylactic and intrathecal therapy for those patients with a relapse because of possible increase in incidence in central nervous system disease and other extramedullary sites of recurrence. Although prophylactic intrathecal therapy has not been proven to prevent central nervous system relapse,59 it may be useful to consider for those patients who present with or who develop leukocytosis during the initial phase of treatment.
Disease-free survival by treatment group.
Reprinted with permission from Shen, et al. Proc Natl Acad Sci, 2004.36
Disease-free survival by treatment group.
Reprinted with permission from Shen, et al. Proc Natl Acad Sci, 2004.36
Comparison of Kaplan-Meier product limit estimate of event-free survival (EFS) between the good-risk group (group A: WBC count, 5 x 109/L; platelet count, 20 x 109/L) and the rest (group B). Reprinted with permission from Mathews et al. Blood. 2006.40
Comparison of Kaplan-Meier product limit estimate of event-free survival (EFS) between the good-risk group (group A: WBC count, 5 x 109/L; platelet count, 20 x 109/L) and the rest (group B). Reprinted with permission from Mathews et al. Blood. 2006.40
Outcome in APL: comparison of APL2000 and LPA 99 in low- and intermediate-risk group. Reprinted with permission from Ades et al. Blood 2008.51
Outcome in APL: comparison of APL2000 and LPA 99 in low- and intermediate-risk group. Reprinted with permission from Ades et al. Blood 2008.51
Outcome in APL: comparison of APL2000 and LPA 99 in high-risk group. Reprinted with permission from Ades et al. Blood 2008.51
Outcome in APL: comparison of APL2000 and LPA 99 in high-risk group. Reprinted with permission from Ades et al. Blood 2008.51
Disclosures Conflict-of-interest disclosure: The authors declare no competing financial interests. Off-label drug use: Gemtuzumab ozogamicin, APL FLT3 inhibitors, decitabine, zarnestra, and arsenic trioxide in the treatment of newly diagnosed APL.
References
Author notes
Northwestern University Feinberg School of Medicine, Robert H. Lurie Comprehensive Cancer Center, Chicago, IL