Abstract
Despite the relatively low incidence of acute lymphoblastic leukemia (ALL) in adults, large national and international collaborations have recently improved our understanding of how to treat ALL in adults. This article documents and examines the current evidence base for a “state of the art” therapy in both Philadelphia chromosome–negative and –positive adult ALL. The article comments upon areas of therapeutic debate, such as the role of bone marrow transplantation. In particular, the controversial subject of whether the superior outcome seen in younger patients is predicated on disease biology or therapeutic strategy is examined closely. Promising approaches under development are also discussed.
Introduction
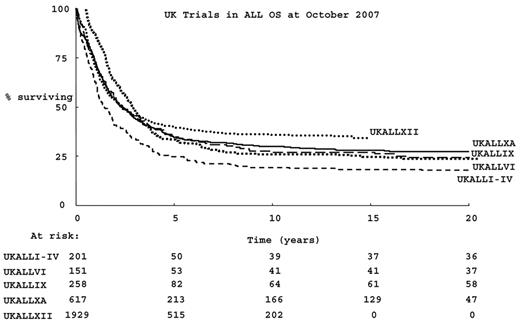
Survival for adults with acute lymphoblastic leukemia (ALL) has improved since the advent of therapy, overall survival (OS) has been better with each successive UK study over the past 35 years, as shown in Figure 1 . However, OS is much less than that of children.1 Initial response rates to combination chemotherapy in adults are almost as high as those seen in children. However, the chance of relapse and the risk of treatment-related mortality are both considerably higher in adults than in children.1 A pressing question is whether the age-related differences in outcome are predicated by the biology of the disease per se or whether they result from a different approach to, or tolerance of, therapy.
There are evident changes in the biology of the disease with age. Most notably, the incidence of “very high risk” cytogenetic categories, such as Philadelphia chromosome–positive (Ph+) ALL, increases with advancing age. Ph+ ALL accounts for approximately 25% of adult ALL2 while it is uncommon (approximately 3%) in children.3 However, there is a body of retrospective evidence suggesting that a “pediatric” approach to therapy gives superior survival an adult approach in adolescents with ALL.4 As a result, the lower limit of “adult” in relation to ALL therapy is no longer clearly defined. “Pediatric” approaches are currently being studied, in a single-arm manner, in patients up to the age of 30 years (Cancer and Leukemia Group-B, USA) or even 50 years (Dana-Farber Cancer Institute, Boston) (see Table 1 for study details). A randomized comparison of adult versus pediatric regimens in the same population has not been done. At the other end of the age continuum, very few studies in adult ALL have included patients over 65 years old; hence, there is no evidence base for an appropriate therapy choice for this age group.
Despite the relatively low incidence of ALL in adults, large national and international collaborations have improved our understanding of how to treat ALL in adults. This article will examine the evidence base for the current “state of the art” of therapy for Ph− and Ph+ adult ALL, as well as comment upon areas of therapeutic controversy and upon promising approaches under development. Where studies are informative in assessing the relative contributions of disease biology versus approach to or tolerance of therapy in the outcome of adult ALL, the findings will be highlighted. Tables 1 and 2 show ongoing Phase II to IV studies in Ph− and Ph+ adult ALL, respectively. Table 3 shows ongoing studies in ALL supportive care. NIH (clinicaltrials.gov) and the European Leukaemia Network websites were searched to provide the information for these tables. In some cases, principal investigators and investigative group leads provided further information. The purpose of the tables is to provide the readers with a general summary of the clinical questions currently being posed in adult ALL.
Induction Therapy in Adult ALL
Ph− ALL
There is broad agreement on the current most appropriate drugs to be used during remission induction in Ph− adult ALL. Regimens comprise steroid, vincristine, and l-asparaginase (l-asp), usually with anthracycline (up to 2 to 3 times the dose given in pediatric protocols) and often with the addition of cyclophosphamide and cytarabine. Comparable rates of complete remission (CR) of approximately 85% to 95% and treatment-related mortality (TRM) are seen in all published regimens. Induction TRM in adults is between 5% and 10%—much higher than the <1% in children.1 Long hospitalizations and serious morbidity during induction are also common. In addition to a predictable relationship between the high doses of anthracyclines and myelotoxicity, there are also numerous instances of unpredictable hepatic and central nervous system and thrombotic toxicities, often l-asp related, which may lead to considerable treatment delays. The decision to continue or resume drugs in the face of toxicities can be a vexing one, since adult ALL is relatively rare and many treating clinicians have not accumulated wide experience with the toxicities of the drug combinations. Furthermore, dealing with individual therapeutic toxicity is almost impossible to “protocolize” within a large clinical trial and points to why promising early results from smaller Phase II studies in adult ALL are rarely recapitulated in the large national and international randomized controlled studies. The appropriate dose, preparation and formulation of l-asp remain unresolved. In pediatric practice, pegylated l-asp (peg-asp) is less immunogenic and gives the most appropriate pharmacokinetic and pharmacodynamic profile,5 but evidence that this agent could be properly used in adults was lacking until a recent CALGB study used peg-asp as part of a multi-agent regimen. Effective asp depletion was achieved in some adults,6 although increasing age was associated with significantly decreased peg-asp doses and less asparagine depletion, providing a clear indication that tolerance of l-asp therapy is age-related.
The most appropriate steroid for induction has arguably been defined as dexamethasone by pediatric practice.7 However, an ongoing Childrens Oncology Group study (AALL0232) is in currently addressing this issue in a randomized comparison. Interesting recent data suggest that dexamethasone pharmacokinetics are extremely variable and that l-asp may impair hepatic synthesis of proteins involved in dexamethasone clearance,8 linking the effectiveness of l-asp therapy with a higher systemic exposure to dexamethasone.
In the face of numerous indications that morbidity and mortality during induction contribute at least in part to the differences in outcome between adult and childhood ALL, supportive care during ALL induction deserves special consideration, yet there are few data on what is optimal and few ongoing studies (Table 3 ). A recent meeting of the European Working Group for Adult ALL documented widely differing recommendations between countries. Particular issues are antifungal prophylaxis and the management of coagulation during l-asp therapy. The risk of fungal infection is high during ALL induction, especially given the high doses of steroids and prolonged myelosuppression, but azoles cannot be given safely in conjunction with vincristine, due to potentiation of neurotoxicity. Confusion regarding the role of coagulation monitoring and factor replacement during l-asp therapy is common. Many protocols for adults recommend routine monitoring and replacement of coagulation factors during l-asp therapy while, in contrast, pediatric protocols often recommend against routine monitoring of coagulation. Interestingly, there is indirect evidence that fresh frozen plasma contains asparagine, which could conceivably compromise l-asp therapy by replenishing the asparagine pool.9
An interesting question in relation to the increased use “pediatric style” regimens in older patients with concomitant exposure to higher doses of steroid is what will happen to the incidence of avascular necrosis (AVN). The first examination of occurrence of AVN in an adult cohort indicated that chemotherapy (as compared with HSCT) and younger age were risk factors—most of the cases occurred in patients below the age of 20 years.10
Ph+ ALL
Until recently, induction therapy in Ph+ ALL did not differ from that for Ph− disease, albeit with significantly lower CR rates. The abl-specific tyrosine kinase (TK) inhibitor, imatinib has now been extensively studied as an addition to the induction therapy of Ph+ ALL although, unfortunately, there have been no randomized controlled studies evaluating imatinib in this setting. In published, non-randomized studies of patients with de novo disease, imatinib appears to improve the CR rate, compared with historical controls. A Japanese Adult Leukemia Study Group (JALSG) showed 96% CR, substantially and significantly higher than that reported in their pre-imatinib trial, although this was a low 51% CR rate.11 More patients therefore received alloHSCT, although short follow-up precluded conclusion about long-term benefit. A small series from the MD Anderson Cancer Center adding imatinib to hyper-CVAD induction also concluded superiority over historical controls treated with chemotherapy alone.12 In older patients, for whom HSCT is not an option, the efficacy of imatinib alone can be studied more clearly. In a German study including patients over 55 years old (median 68 years), imatinib was randomized between co-administration with induction chemotherapy or subsequent co-administration with consolidation chemotherapy.13 The CR rates were 96% and 50%, respectively. However, there was no significant difference between the 2 cohorts in OS. Only 43% of patients had undetectable bcr-abl transcripts; the OS in patients who had undetectable bcr-abl transcripts was superior to the OS of those who did not. The British/North American study UKALLXII/ECOG2993 is still open to recruitment for patients with Ph+ ALL. A recent interim analysis was presented at ASH 2007, comparing patients treated in the pre-imatinib era with those in whom imatinib was introduced into the same treatment regimen. There was no OS advantage for those patients whose regimen included imatinib. However, the data from this study will not be fully mature for some time, so final conclusions are not yet possible. A German study examined the potential mechanism for the lack of sustained efficacy with imatinib; Pfeifer et al14 reported that many patients harbored a small leukemic clone—below the level of detection of direct cDNA sequencing—with a kinase domain mutation at diagnosis. Whilst the existence of this clone did not affect either the CR rate, there was a suggestion that relapse was considerably more frequent among patients presenting with kinase domain mutations. Abnormal TK activity alone is not entirely responsible for the phenotype of Ph+ ALL (unlike CML) and in ALL Src kinase activity is involved.15 Hence, clinical data and the theoretical basis for the development of Ph+ ALL suggest that it remains possible that the improved initial responses to imatinib will not translate into improved survival. Simultaneous inhibition of both tyrosine and Src kinases might hold out more promise than TK inhibitors alone. Dasatinib, an inhibitor of both bcr-abl and Src family kinases is an obvious candidate drug.
A recent Phase II study assessed the efficacy, safety, and tolerability of dasatinib in 36 patients with Ph+ ALL who were resistant to or intolerant of imatinib.16 Major hematological responses were achieved in 42% of patients (duration 1.9 to 8.7 months), 67% of whom remained progression-free. Over half the patients attained a complete cytogenetic response. Importantly, the presence of bcr-abl mutations conferring imatinib-resistance did not preclude a response to dasatinib. Current data support a role for TK inhibitors both during induction and post HSCT. However, no randomized studies have been carried out and long-term benefits remain unclear.
New drugs for induction
Intensity of induction therapy, particularly myelo-suppression, is at the limit of tolerance for adults with ALL. If new drugs are to be used in induction, toxicities must not recapitulate those of current therapy. Obvious candidates for addition to induction therapy are monoclonal antibodies, which are being investigated by several groups in large randomized controlled trials (RCTs) (see Table 1 for ongoing studies in Ph− ALL). For patients with T-cell ALL, nelarabine is the most obvious candidate drug for larger study, with good evidence for efficacy in a Phase II study of relapsed disease.17 A number of drugs are being studied in early phase trials and these are recently well reviewed.18
Prognostic factors in adult ALL
Standard prognostic factors in adult ALL
Prognostic factors are considered in the section on induction therapy because their typical place in current adult ALL treatment is in directing post-remission therapy. A number of de-novo prognostic factors in adult ALL are very clearly defined—age (although clearly a continuum, 35 years appears to be a clear prognostic cut-off), presenting white cell count (> 30 × 109/L for B-cell disease and > 100 × 109/L for T-cell disease), immunophenotype (T-cell disease has a better outcome than B-cell disease in adults) and Ph status and have recently been confirmed in a large study.19 Many studies in adult ALL already use one or more of these prognostic factors in determining the course of therapy after induction. However, it is important to recall that there is no clear evidence that increasing the intensification of post-remission therapy—using either chemotherapy or alloHSCT—can alter the outcome of poor-risk disease.
Cytogenetics in adult ALL
This year, two landmark studies have further defined cyto-genetic risk factors for adult ALL. In a review of 1522 adult patients from the UKALLXII/ECOG2993 study,2 patients with Ph chromosome, t(4;11), complex karyotype (defined as 5 or more chromosomal abnormalities), or low hypodiploidy/near triploidy had inferior rates of event-free survival and OS when compared with other patients. Among patients with Ph− ALL, the prognostic relevance of complex karyotype and low hypodiploidy/near triploidy was independent of the known prognostic factors listed above. This was the first demonstration that cytogenetic subgroups other than Ph chromosome can be used for risk-stratification of adults with ALL. A report from SWOG-940020 on the outcome of 200 patients (140 evaluable for cytogenetics) in relation to cytogenetic abnormalities recapitulated the findings of the UKALLXII/ECOG2993 study and a previous GIMEMA study21 and, importantly, suggested that when the effect of cytogenetics on OS was accounted for, age was not a significant prognostic factor. This suggests that the worsening prognosis with advancing age in adult ALL could at least in part be a manifestation of the age-related increase in unfavorable cytogenetics.
Minimal residual disease in adult ALL
Both molecular and immunophenotypic methods can be used to reliably detect the presence of residual ALL at levels of less than 0.01%. In adults, identification of patient-specific immunoglobulin and T cell receptor (Ig/TCR) gene re-arrangements and subsequent real-time PCR quantitation of selected targets predominates, with the role of flow cytometry less well studied in this context. A good quality diagnostic sample, usually bone marrow, is an absolute requirement for both approaches. Bone marrow specimens are required during follow-up to achieve optimal sensitivity. In the laboratory, finding a marker and determination of a useful quantitative range at an appropriate level of sensitivity depends very heavily upon experienced technical staff. The European Study Group (a group of 30 ALL minimal residual disease [MRD] laboratories) published guidelines on determination and interpretation of MRD by Ig/TCR gene re-arrangements,22 which are intended to facilitate interpretation of MRD results from different studies. Technical considerations aside, the predictive value of MRD at a given time-point is protocol specific. Seminal work on MRD in adult ALL has been carried out by GMALL, the German study group. Bruggemann et al23 prospectively monitored MRD in 196 “standard-risk” patients and within this seemingly homogenous group, identified—at week 16 of therapy—a subset of patients at particularly high risk of relapse (RR 94%). Very few adults had a rapid decline of MRD to less than 10−4 at day 11. Only in 10% of individuals could MRD be used to predict a very good outcome, but among those who had no evidence of MRD at day 11 and 24 no relapse occurred. Another key role for MRD monitoring would be if it allowed the opportunity to intervene earlier in the case of impending relapse. Another GMALL study24 systematically examined the occurrence of MRD, within the quantitative range, during consolidation and maintenance, carrying out the test on 3-monthly bone marrow specimens. Twenty-two of 105 previously MRD-negative patients relapsed after the first year of therapy. In 17 of these, MRD was detected before relapse, while in 5 patients MRD was not detected before relapse. It is currently unclear whether early detection of MRD can offer a realistic opportunity to intervene clinically and alter the course of the disease but this merits careful study.
Consolidation, Intensification, CNS-Directed Therapy and Maintenance Therapy
Consolidation/maintenance therapy for adults is based on that used in pediatric regimens. No randomized studies usefully address the benefits of the number or composition of consolidation cycles in adult ALL. Intensification with high-dose methotrexate (MTX) 1.5 to 3 g/m2, sometimes in conjunction with l-asp, is commonly used and is an important component of central nervous system (CNS)-directed therapy.
Although CNS involvement is uncommon at diagnosis in adults (5%), good outcomes can still be achieved in this setting.25 Isolated CNS relapse is also uncommon —approximately 3% in patients relapse after the UKALLXII/ ECOG2993 protocol.26 Intrathecal therapy is also vital in preventing and treating CNS leukemia. There are no data on the most efficacious drug or drug combination in adults, although in children RCT evidence demonstrated a decrease in CNS relapse with triple MTX, cyatarabine and hydrocortisone by comparison with MTX alone. The possibility of a reduction in the number of lumbar punctures required was attractive, but when a long-acting liposomal cytarabine was used in combination with Hyper-CVAD, severe unexpected neurotoxicity occurred in 16% of patients, particularly when the drug was given concomitantly with systemic MTX and cyatarabine at levels that cross the blood-brain barrier.27
The role of cranial irradiation in providing CNS-directed prophylaxis in adults, now that effective local and systemic therapy is available, is difficult to gauge. In the pediatric population, cranial irradiation is now used very infrequently. In adults the dual concerns of toxicity and of lack of systemic therapy during CNS irradiation, coupled with the increased application of alloHSCT in which total-body irradiation (TBI) is used are leading investigators to also consider evaluating omitting CNS irradiation. CNS relapse is now sufficiently infrequent as to render the numbers of patients required to evaluate this in a randomized manner pragmatically impossible, so studies can only aim to compare CNS relapse rates with that in historical controls, and careful interim analyses are needed.
Maintenance therapy remains obligatory in those not undergoing alloHSCT. Daily mercaptopurine, weekly MTX and pulses of vincristine and steroids for 18 to 24 months after consolidation is standard. There is now RCT evidence of the value of prolonged chemotherapy in adult ALL. In UKALLXII/ECOG2993, patients without a matched sibling donor were eligible for randomization between high-dose therapy with etoposide and TBI with autologous HSCT (autoHSCT) rescue and maintenance therapy. The intent-to-treat analysis of 456 randomized patients showed that those randomized to prolonged chemotherapy had significantly superior event-free survival (41% vs 32%; P = .02); and OS (46% vs 37%; P = .03) at 5 years, compared with those randomized to autoHSCT. It is of particular interest that the TRM did not differ between the groups, Hence, autoHCST simply provided less-adequate disease control than prolonged chemotherapy.
Bone Marrow Transplantation
Role of myeloablative alloHSCT in Ph− ALL
Myeloablative sibling donor alloHSCT has long been used in the treatment of adult ALL. It is worth noting that there has never been a successful randomized trial of allogeneic HSCT and that the so-called “biological randomization” represents the gold standard with which to evaluate alloHSCT. A long-awaited analysis of a the largest donor versus no donor comparison in adult ALL was recently published.28 The UKALLXII/ECOG2993 study allocated all patients with a fully matched sibling donor to etoposide and TBI-conditioned alloHSCT. Although not recommended in the protocol, the final decision on T-cell depletion was left to treating centers. Those without a donor were eligible to enter a randomized comparison of autoHSCT versus maintenance chemotherapy. An intention-to-treat analysis of the entire patient cohort, showed that patients with a donor had a 5-year OS of 53% versus 45% for those without a donor (P = .01) with an associated significantly lower relapse rate (P ≤ .001). Notable was the observation that the benefit of alloHSCT was apparently confined to those with “standard risk” disease. Despite a highly significant reduction in relapse risk in both standard-risk (49% no donor vs 24% donor) and high-risk patients (63% no donor vs 37% donor), P < .00005, the TRM among “high-risk” patients was sufficient in magnitude to abrogate a survival advantage in this high risk group despite the evident anti-leukemia activity of the procedure indicated by the reduction in relapse risk. Since one of the criteria for “high risk” is age greater than 35 years and given that advancing age also confers the highest risk of TRM, this is probably the main reason for lack of survival advantage in the “high-risk” group, although the subgroup numbers do not allow formal statistical proof within this dataset. Results from the LALA94 study similarly suggest a survival advantage to sibling alloHSCT in patients with high-risk cytogenetic abnormalities such at t (4; 11).29 Two meta analyses conducted on abstracted data30,31 also evaluated alloHSCT in ALL in CR1 and concluded that there was an advantage to alloHSCT, even in those with high-risk disease—and that this advantage was cost effective. Meta analyses conducted on primary data may be the only way to answer some of the remaining complex questions for which individual clinical trials of alloHSCT could never be expected to accrue sufficient patient numbers.
There are few published data on optimal conditioning regimens but TBI is a key component. An IBMTR study suggested a benefit to the combination of etoposide with TBI.32 The role of T-cell depletion is also unclear—practice in this area tends to be institutionally or nationally based. Unrelated donors are increasingly used as a source of stem cells, and data suggest little difference in the outcome, given good matching.33 With increasing use of unrelated donors, the true role of alloHSCT may become almost impossible to evaluate formally.
Reduced-intensity conditioned alloHSCT
Since the age threshold at which TRM exceeds reduction in relapse risk may be as low as 35 to 40 years old, it is very reasonable to look to reduced-intensity conditioned HSCT as a way to provide a graft-versus-leukemia effect with reduced toxicity. The success of this approach is likely to be disease burden dependent; absence of MRD at the time of transplant may be of crucial importance although this has not been formally studied. reduced-intensity conditioned HSCT has been described in several retrospective series,34-36 all of which have included patients with both Ph+ and Ph− disease and patients with ALL beyond CR1. The largest series to date is an EBMT study of 97 patients who received a mixture of conditioning regimens and more than one-third received some form of T-cell depletion. A 2-year OS of 52% for those transplanted in CR1 was reported. This approach merits consideration, but careful prospective study is still required to define its role.
AlloHSCT in Ph+ ALL
Ph+ ALL has long been considered such a high-risk disease that alloHSCT has been assigned to all eligible patients. This may have obviated the opportunity to carefully study the role of this approach. However, accumulated evidence of the very poor results of treating this disease with chemotherapy alone, accompanied by reports from retrospective series of alloHSCT suggest that myeloablative therapy, with a TBI-based conditioning regimen followed by sibling alloHSCT, represents the current best available treatment option for appropriately aged patients with Ph+ ALL in CR1. The role of HSCT in Ph+ ALL has recently been comprehensively reviewed.37 The largest prospective study of unrelated donor HSCT in de novo adult Ph+ ALL was carried out in UKALLXII/ECOG2993. At 5 years there was no statistically significant difference in OS or in cause of death between those receiving sibling alloHSCT and those receiving matched unrelated donor alloHSCT. Whereas the leading cause of death in chemotherapy-treated patients was relapse, the leading cause of death after alloHSCT was related to treatment (AK Fielding, SM Richards, JM Rowe and AH Goldstone, personal communication).
AutoHSCT
Although a individual data-based overview of the last three trials from the LALA group suggested some utility,38 recent the RCT evidence discussed above leaves little role for the routine application of autoHSCT in adult ALL.
Treatment of Relapsed ALL
Two large studies on the treatment of relapsed ALL in adults have recently confirmed that, in most cases, salvage after relapse is not feasible.26,39 The worst prognosis from relapse occurs with the shortest duration of CR1 and in older patients. These studies are important not only in determining realistic approaches to salvage therapy for individuals but also to confirm that prevention of relapse is the highest priority in adult ALL therapy and that it is not inappropriate to study the impact of high-risk strategies in relapse prevention in those at highest risk of relapse.
Scientific Insights Likely to Impact Future Therapy
The past year has seen some major scientific insights into ALL biology. The known chromosomal aberrations in ALL are not causative, so the question of what cooperating oncogenic lesions are required is a vital one to answer. A major study from the St Jude’s group carried out a comprehensive registry of genetic lesions in ALL using single nucleotide polymorphism (SNP) arrays. Fifty-four recurrent regions of deletion were identified; this study generated a number of candidate genes were identified for further study.40 Fascinating work from several groups has focussed on the existence of ALL stem cells. Particularly thought provoking is work from a study of twins discordant for ALL, which demonstrated the function of TEL-AML1 as a “first hit” mutation generating a pre-leukemic population with altered self-renewal and survival properties from which ALL can arise.41,42 Also of interest was work suggesting a role for stromal cell support in maintaining ALL—mesenchymal stem cells were able to secrete asparagine synthetase to protect ALL blasts against toxic levels of l-asp.43
Successive UK trials in adult acute lymphoblastic leukemia (ALL) in the past 35 years: overall survival.
Successive UK trials in adult acute lymphoblastic leukemia (ALL) in the past 35 years: overall survival.
Disclosures Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
References
Author notes
Royal Free and University College Medical School, London, UK