Abstract
Paroxysmal nocturnal hemoglobinuria is a clonal hematopoietic stem cell disease that manifests with intravascular hemolysis, bone marrow failure, thrombosis, and smooth muscle dystonias. The disease can arise de novo or in the setting of acquired aplastic anemia. All PNH patients to date have been shown to harbor PIG-A mutations; the product of this gene is required for the synthesis of glycosylphosphatidylinositol (GPI) anchored proteins. In PNH patients, PIG-A mutations arise from a multipotent hematopoietic stem cell. Interestingly, PIG-A mutations can also be found in the peripheral blood of most healthy controls; however, these mutations arise from progenitor cells rather than multipotent hematopoietic stem cells and do not propagate the disease. The mechanism of whereby PNH stem cells achieve clonal dominance remains unclear. The leading hypotheses to explain clonal outgrowth in PNH are: 1) PNH cells evade immune attack possibly, because of an absent cell surface GPI-AP that is the target of the immune attack; 2) The PIG-A mutation confers an intrinsic resistance to apoptosis that becomes more conspicuous when the marrow is under immune attack; and 3) A second mutation occurs in the PNH clone to give it an intrinsic survival advantage. These hypotheses may not be mutually exclusive, since data in support of all three models have been generated.
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) was originally recognized as a hemolytic anemia. Following the emergence of specific diagnostic tests, additional disease manifestations such as venous thrombosis, bone marrow failure and development of myelodysplastic syndromes and acute leukemia were associated with PNH. These non-erythroid manifestations foreshadowed the discovery that PNH arises from the clonal expansion of a mutated hematopoietic stem cell. In the early 1990s it was demonstrated that GPI anchor protein (GPI-AP) deficiency was the consequence of a somatic mutation in the X-linked gene known as PIG-A. The product of this gene is required for the biosynthesis of GPI anchors. Thus, PNH blood cells have a cell surface deficiency of all proteins that use the GPI anchor for attachment to the cell membrane. The median survival for PNH patients is 10 to 15 years after diagnosis; however, the recent advent of eculizumab, a novel monoclonal antibody that decreases hemolysis and thrombosis in PNH, may alter the natural history.1,2 Prior to eculizumab, thrombosis was the leading cause of death, but additional patients died from complications of bone marrow failure, kidney failure, or malignant transformation. Thus, PNH remains at the interface between a benign disease and low-grade malignancy of hematopoietic stem cells.
The PIG-A Gene
The human PIG-A gene contains 6 exons, 5 introns and extends over 17 kb; it codes for a protein that contains 484 amino acids (60 kDa). In humans, there is a single copy of the gene located on the short arm of the X chromosome (Xp22.1). A wide range of somatic mutations interspersed throughout the entire coding region of the PIGA gene have been described in PNH patients. There are no true mutational “hotspots,” although exon 2, which contains almost half of the coding region, is the exon where most mutations occur. Most PIG-A mutations are small insertions or deletions that result in a frameshift in the coding region and consequently a shortened, non-functional product. While PIG-A function is abolished by these frameshift mutations, missense mutations, where the product of the mutated PIGA gene has some residual activity, have also been described. In most patients studied, a single (monoclonal) PIGA mutation has been discovered. However, 2 different mutations (biclonal) and in one case 4 separate PIGA mutations have been found in PNH patients.3
The PNH Stem Cell
PNH is a clonal hematopoietic disorder similar to the myelodysplastic syndromes and the myeloproliferative disorders. The first evidence to support the notion that PNH arises through the mutation of an abnormal multipotent hematopoietic stem cell was derived from glucose 6-phosphate dehydrogenase studies on the red cells of women with PNH.4 Subsequently, flow cytometric analyses revealed that all hematopoietic lineages—myeloid, erythroid, and lymphoid—were involved. Furthermore, PIGA mutations found in granulocytes match those found in other lineages,5,6 and CD34+CD38− progenitor cells have been shown to be missing GPI-AP in PNH patients.7 Thus, the “hit” in PNH clearly involves a multipotent hematopoietic stem cell and appears to involve an earlier stem cell than diseases such as chronic myelocytic leukemia, MDS, or acute leukemia. In the latter disorders, B cells are sometimes derived from the leukemia clone, but T cells are seldom involved. In PNH, both B cells and T cells have been shown to be derived from the mutated hematopoietic stem cell.
PIG-A Mutations in Aplastic Anemia and MDS
Small to moderate PNH clones are found in up to 70% of patients with acquired aplastic anemia demonstrating a pathophysiologic link between these disorders.8–11 Typically, less than 20% GPI-AP–deficient granulocytes are detected in aplastic anemia patients at diagnosis, but occasional patients may have larger clones.10 DNA sequencing of the GPI-AP–deficient cells from aplastic anemia patients reveals clonal PIGA gene mutations.12 Moreover, many of these patients exhibit expansion of the PIGA mutant clone and progress to clinical PNH. While it was once thought that PNH evolving from aplastic anemia is more benign than classical PNH, this observation may be a consequence of lead time bias, since many of these patients eventually develop classical PNH symptoms.
GPI-AP–deficient cells have also been reported in patients with MDS,9,11 but sequencing of the PIGA gene to establish clonality has not been performed in many of these studies. Patients with MDS who are reported to have small PNH populations tend to be classified as having refractory anemia and often have the following characteristics: a hypocellular marrow, HLA-DR15 positivity, normal cytogenetics, moderate to severe thrombocytopenia, and a high likelihood of response to immunosuppressive therapy.11,13 Thus, it is possible that many of these patients have moderate aplastic anemia rather than MDS. Distinguishing hypoplastic MDS from aplastic anemia is often difficult; however, quantitative analysis of bone marrow CD34+ cells is useful for discriminating between these two entities.14
Disruption of PIG-A in Human and Murine Embryonic Stem Cells
To establish an experimental system to study PNH, murine models have been established by disrupting the Pig-a gene in mouse embryonic stem (mES) cells.15 Undifferentiated mES grow normally in culture; however, upon differentiation these cells do not form embryoid bodies. Thus, disrupting Pig-a is embryonic lethal. Conditional Pig-a null mice lacking GPI-APs in all hematopoietic lineages have been established; however, these mice have a normal life span and do not recapitulate signs of the human disease. Specifically, they do not show evidence of intravascular hemolysis, thrombosis, smooth muscle dystonias or bone marrow failure.16,17
Recently, we disrupted PIG-A in a human embryonic stem (hES) cell.18 Unlike the Pig-a null mES cells, PIG-A null hES cells are capable of forming embryoid bodies and initiating differentiation into all three embryonic germ layers—endoderm, ectoderm and mesoderm. However, GPI-AP–deficient hES cells failed to form trophoblasts after differentiation induction by embryoid body formation or by adding exogenous bone morphogenic protein-4 (BMP4). The defect in trophoblast formation was due to the lack of GPI-anchored BMP coreceptors, principally DRAGON, resulting in the perturbation of full BMP4 signaling activation in the GPI-AP–deficient hES cells. These data demonstrate that GPI-AP–enhanced full activation of BMP signaling is required for human trophoblast formation. These data underscore that important differences may occur between mES and hES and demonstrate that the absence of a single cell surface GPI-AP may have a profound effect on cell growth and differentiation.
PIG-A Mutations in “Normal” Hematopoiesis
PNH is a rare disease. The incidence is estimated to be 2 to 5 new cases per million US inhabitants annually; however, PIGA mutations can be found in the blood from virtually all healthy control subjects.6,19,20 Araten and colleagues found an average of 22 GPI-AP–deficient granulocytes per 106 cells. GPI-AP–deficient lymphocytes have also been detected in patients with lymphoid malignancies or rheumatoid arthritis after treatment with alemtuzimab.21–23 Alemtuzimab is a monoclonal antibody that induces cell death in cells (generally, monocytes and lymphocytes) expressing the GPI-AP, CD52. Several weeks after the administration of alemtuzimab GPI-AP–deficient lymphocytes often emerge, but promptly regress after discontinuation of the drug. Thus, alemtuzimab leads to selective survival of a PNH-like population of cells in non-PNH patients.
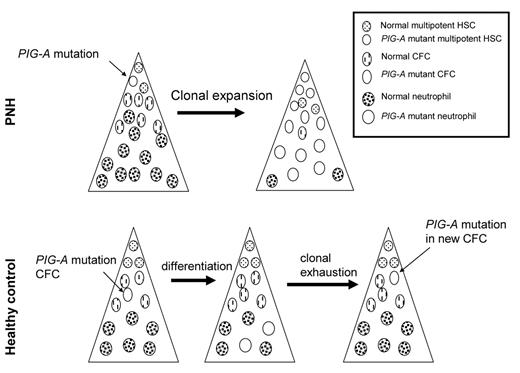
How can such a commonly mutated gene, PIG-A, be so specific for PNH, yet so rarely result in disease? One possibility is that PIG-A mutations are not sufficient to cause PNH. Alternatively, it is possible that PIG-A mutations in healthy control subjects do not occur in a multipotent hematopoietic stem cell. Moreover, these hypotheses are not mutually exclusive. To determine whether multipotent hematopoietic stem cells from healthy donors harbor PIG-A mutations, we isolated CD34+ cells from PNH patients and healthy control subjects. The frequency of PIG-A mutant progenitors was determined by assaying for colony forming cells (CFC) in methylcellulose containing toxic doses of proaerolysin.6 GPI-APs serve as receptors for proaerolysin; thus, PNH cells are resistant to the toxin, whereas cells expressing GPI-AP are killed almost immediately by proaerolysin.24 The frequency of proaerolysin-resistant CFC was 1 in ~65,000 from healthy controls and approximately 1 in 2 from PNH patients. DNA was extracted from individual (plucked) proaerolysin-resistant colonies and the PIGA gene from each colony was sequenced. All of the proaerolysin resistant colonies from each PNH patient contained the same PIG-A mutation (indicating that they were “clonal” progeny of a PIG-A-mutated pre-CFC); and, in each patient with PNH, the same mutation was found in the CFC of all lineages (CFU-GM, CFU-GEMM, BFU-E), as well as in T lymphocytes. In contrast, the PIG-A mutations we found in the hematopoietic colonies from CD34+ cells from healthy control individuals indicated “polyclonality” of the PIG-A–mutated cells (i.e., the mutations found in 2 or more myeloid and/or erythroid colonies were not the same). Furthermore, T cells from these patients did not harbor matching PIG-A mutations. In one patient we found over a dozen different PIG-A mutations. These data suggest that most PIG-A mutations in hematopoietic cells from healthy controls occur during differentiation (Figure 1 ). DNA repair is progressively attenuated during the process of cellular differentiation,25 possibly leading to differentiation-dependent spontaneous mutations.26PIG-A mutations occurring in cells that lack self-renewal capacity (e.g., CFC) will not propagate disease since the average lifespan of these cells is roughly 125 days.27 This explains why PIG-A mutations in more differentiated cells (e.g., granulocytes) are common in healthy control subjects; however, these data do not explain how clonal expansion of a PIG-A mutant stem cell occurs in patients with PNH.
Clonal Expansion in PNH
PNH originates from a multipotent hematopoietic stem cell. It is now clear that most, if not all, PIG-A mutant cells in healthy controls arise from CFC.6,28 However, it is not clear that PIG-A mutations that occur in a hematopoietic stem cell are sufficient to cause the disease. The mechanism of clonal expansion remains an enigma. Three leading models/hypotheses have been proposed to explain clonal expansion of the PNH stem cell. These models are not mutually exclusive; all of them have attractive features as well as potential pitfalls.
Model 1:
PNH cells evade immune attack, possibly because a missing cell surface GPI-AP that is the target of the immune attack.29 This model is attractive for explaining the close association between acquired aplastic anemia and PNH; however, no GPI-AP has been shown to be the target of an immune attack in aplastic anemia or PNH. Furthermore GPI-AP are translated in PNH cells but are degraded intracellularly if the GPI anchor is not synthesized;30 therefore, protein epitopes should be displayed extracellularly by histocompatibility antigens and be immunogenic even if the intact protein is not on the cell surface. Hanaoka and colleagues have proposed an alternative mechanism whereby a missing cell surface protein could lead to a clonal advantage in the setting of an immune attack.31 These authors found that preferential survival of the PNH clone may be attributable to the deficiency of stress-inducible GPI-APs ULBP1 and ULBP2. ULBP-1, -2, and -3 were originally identified as ligands for the human cytomegalovirus glycoprotein UL16 and designated UL16-binding proteins (ULBP). ULBPs are expressed when cells are under stress; they serve as receptors for NK- and T-cell–mediated killing. Thus, it is possible that PNH cells with an absence of stress-inducible membrane proteins such as ULBPs would preferentially survive an immune-mediated attack.
Model 2:
The PIG-A mutation itself confers an intrinsic resistance to apoptosis32 that becomes more conspicuous when the marrow is under immune attack. No survival advantage has been found under a variety of conditions in the mouse model of PNH,33 but several studies have found that primary GPI-AP–deficient myeloid cells from PNH patients are relatively resistant to apoptosis.32,34–37 Nevertheless, two of these studies concluded that, since the degree of resistance was not proportional to PNH clone size, the resistance may be independent of the PIG-A mutation.35,37 Because primary GPI-AP–deficient CD34+ progenitors exhibited similar proliferative and survival rates to CD34+ progenitors from healthy controls, these studies concluded that the survival advantage of the GPI-AP–deficient cells resulted from diminished survival of GPI-AP+ stem cells in a “hostile” PNH environment.34,36,38 However, it is possible that GPI-AP–deficient stem cells may also be damaged by the same hostile environment, and an intrinsic survival advantage in PNH cells may be obscured when PNH progenitors are compared to normal control cells.
Model 3:
A second anti-apoptotic mutation occurs in the PNH clone.39 Support for this hypothesis is derived from a recent report of 2 patients with PNH in whom an acquired rearrangement of chromosome 12 produced ectopic expression of the HMGA2 gene. HMGA2 is deregulated in benign mesenchymal tumors (e.g., lipomas) and was overexpressed at the mRNA level in the PNH clone of these patients. It is possible that deregulated HMGA2 expression in concert with a PIGA mutation accounted for the clonal expansion in these 2 patients. However, subsequent studies involving 42 patients with PNH did not reveal an increase in HMGA2 mRNA, suggesting that deregulation of HMGA2 is not a major mechanism to explain clonal expansion in PNH.40 It remains possible that another, presently unrecognized mutation or survival factor, is required for clonal expansion.
Summary
The mechanism of clonal dominance in PNH remains a mystery. In spite of the fact that PNH cells are more vulnerable to complement-mediated killing, somehow a multipotent hematopoietic stem harboring a PIG-A mutation manages to survive better than normal hematopoietic stem cells. Improved understanding of this process will likely give greater insight into the mechanism(s) of clonal dominance in other hematopoietic stem cell diseases such as MDS.
PIG-Amutations in health and disease.PIG-A mutations in PNH (upper panel) arise from a multipotent hematopoietic stem cell (HSC). Expansion and differentiation of the PIG-A mutant HSC results in clinical disease. In healthy control subjects, most, if not all, PIG-A arise from colony-forming cells (CFC). CFC can differentiate, but have no self-renewal capacity; hence, these PIG-A mutations do not result in disease.
PIG-Amutations in health and disease.PIG-A mutations in PNH (upper panel) arise from a multipotent hematopoietic stem cell (HSC). Expansion and differentiation of the PIG-A mutant HSC results in clinical disease. In healthy control subjects, most, if not all, PIG-A arise from colony-forming cells (CFC). CFC can differentiate, but have no self-renewal capacity; hence, these PIG-A mutations do not result in disease.
Disclosures Conflict-of-interest disclosure: The author declares no competing financial interests. Off-label drug use: None disclosed.
References
Author notes
Division of Hematology, Johns Hopkins University School of Medicine, Baltimore, MD