Abstract
Cure of acute promyelocytic leukemia (APL) is now a possibility for most patients through the use of state-of-the-art treatments, which include simultaneous administration of all-trans retinoic acid (ATRA) and anthracycline-based chemotherapy for induction and consolidation, as well as ATRA-based maintenance. Risk-adapted strategies to modulate treatment intensity may be an effective approach to minimize therapy-related morbidity and mortality while maintaining the potential of cure. In this context, there is no role for hematopoietic stem cell transplantation (HSCT) in front-line therapy, except for the small fraction of patients with persistent minimal residual disease at the end of consolidation. However, HSCT plays an important role for patients in second complete remission. In contrast, an increasing role of arsenic trioxide (ATO) is emerging. Given the high antileukemic efficacy observed with ATO in patients relapsing after ATRA-containing regimens, this agent is currently regarded as the best treatment option in this setting. However, until a randomized comparison between the standard therapy and ATO-based regimens in front-line therapy is available, this latter approach should only be recommended for unfit patients for whom chemotherapy is contraindicated. In addition to reviewing current consensus and controversial issues on antileukemic strategies, this review addresses other aspects that can be crucial for the outcome of individual patients. These aspects include supportive care, recognition and treatment of life-threatening complications, evaluation of response, and, finally, management of the disease in special conditions such as older patients, children and pregnant women.
Since the first description of acute promyelocytic leukemia (APL) in 1957 as the most malignant form of acute leukemia,1 several developments have paved the way to make this disease the most curable leukemia in adults and change the paradigm of cancer treatment. Therapy of APL was pioneered by Bernard et al in 1973 with a seminal contribution demonstrating a striking sensitivity to daunorubicin,2 probably related to significantly lower P-glyco-protein expression observed in APL cells compared to other subtypes of acute myeloid leukemia (AML).3 Prior to the incorporation of all-trans retinoic acid (ATRA), following a revolutionary contribution of the Shanghai group in 1988,4 treatment of APL was essentially based on two main approaches—an AML-type and an APL-adapted chemotherapy. The latter approach, generally adopted by European investigators, used daunorubicin or idarubicin as single agents, while the former combined these and other anthracyclines to cytarabine.5 Several reports confirmed that daunorubicin and idarubicin as single agents induced complete remission (CR) in 55%–88% of patients.5,6 These CR rates appeared comparable to those reported with combination chemotherapy5 and the only randomized study comparing both approaches in the pre-ATRA era also found no statistically significant differences.7 The incorporation of ATRA, a non-cytotoxic differentiating agent that is regarded as the first paradigm of molecularly targeted therapy, has changed dramatically the management, outcome, and prognosis of APL, especially when ATRA is combined with anthracycline-based chemotherapy. More recently, arsenic trioxide (ATO) has been included in the armamentarium of active drugs in APL, being perhaps the most active single agent.8
This review covers current strategies for the treatment of APL and highlights management issues encountered in the clinical practice that can be crucial for the outcome of individual patients. The potential role and place of newer agents in both front-line and salvage therapy will also be discussed.
InductionTherapy for Newly Diagnosed APL Patients
The current standard approach: ATRA plus anthracycline-based chemotherapy
Initial studies on ATRA monotherapy, which resulted in high rates of CR, made evident the need for administering some type of consolidation chemotherapy to avoid disease relapse. Several studies conducted in the early 1990s, and especially two randomized trials of the European APL group9 and the North-American Intergroup,10 showed that patients receiving ATRA followed by chemotherapy had significantly better outcomes as compared to patients treated with chemotherapy alone.5,6 In both studies, the CR and early death rates were not statistically different, but the relapse rate was significantly higher for the patients treated with chemotherapy alone. However, the outcomes with the sequential administration of ATRA followed by chemotherapy were subsequently improved on over the past decade when ATRA plus chemotherapy was given simultaneously. This was clearly shown in a randomized study of the European APL group11 comparing the sequential versus the simultaneous ATRA plus chemotherapy schedule, and further confirmed in other large multicenter trials (Table 1 ).12–17 Based on these studies, a consensus has been reached to establish the combination of ATRA plus chemotherapy as the current standard approach for newly diagnosed APL.6,18
As to the type of anthracycline and whether it should be combined with other agents, both issues still remain controversial, at least as far as induction therapy is concerned. A recent randomized trial of the European APL group19 demonstrated an increased risk of relapse when cytarabine was omitted from a schedule using daunorubicin as anthracycline (findings to be discussed in the post-remission therapy section). However, this study was unable to demonstrate differences in terms of CR or induction failure rates. It should also be noted that when response is appropriately assessed, virtually no cases of leukemia resistance are reported using ATRA and idarubicin.17,20 With respect to the type of anthracycline, idarubicin has shown a slight survival advantage when compared with daunorubicin in conjunction with cytarabine only in younger AML patients.21 In APL, no prospective studies have been conducted to assess the comparative value of both anthracyclines.
Clinicians are frequently tempted to modify the standard approach with ATRA and anthracycline-based chemotherapy based on supposedly “adverse” prognostic factors such as additional chromosome aberrations other than t(15;17), CD56 expression, or short PML/RARα isoform. However, in large cohorts of patients receiving modern ATRA plus chemotherapy regimens, none of these factors has been shown to affect the prognostic outcome.18
Role of arsenic trioxide in front-line therapy
Following the successful results in the treatment of relapsed patients with APL first reported in China and then replicated in Western populations,22,23 which observed remission rates of more than 80% and high rates of molecular remission, several trials have been designed to investigate the role of ATO in front-line therapy (Table 2 ). Using ATO for induction in newly diagnosed APL, Shen et al24 reported on 61 patients who were randomized into three treatment groups: ATRA, ATO, and the combination of the two drugs. CR rates were high in the three arms (≥90%), but with the combination treatment the time to achieve CR was significantly shorter and the reduction of the disease burden as assessed with molecular methods was greater. More recently, in a series of 44 patients, including ATRA plus ATO, with the addition of gemtuzumab ozogamicin for high-risk patients, resulted in 89% CR rate (96% and 79% for standard and high-risk patients, respectively).25 Two additional studies from India26 and Iran,27 with ATO as a single agent, reported the same CR rate of 86% in 72 and 94 newly diagnosed patients, respectively.
In spite of these promising results, the current recommendation for induction therapy in newly diagnosed APL is the standard approach with ATRA plus chemotherapy. Lacking an appropriate comparison between the standard induction therapy and ATO-based regimens in a randomized clinical trial, this latter approach should only be recommended for unfit patients for whom chemotherapy is contraindicated.
Supportive measures during induction therapy
In addition to the specific antileukemic treatment, supportive care and other therapy-related issues are also critical for the successful outcome in individual patients.18 Once the suspicion of APL is established on the basis on morphologic criteria, the disease should be managed as a medical emergency, starting supportive measures and ATRA therapy even before the genetic diagnosis is available. The importance of rapidly providing adequate supportive therapy relies on the fact that a sizable fraction of patients develop fatal hemorrhages during the diagnostic evaluation, before beginning antileukemic therapy, or during the first days of induction. It seems reasonable, therefore, that rapid initiation of ATRA and supportive measures to reverse the ongoing coagulopathy may lower the risk of life-threatening hemorrhages in these patients. Treatment of the coagulopathy should be based on liberal transfusion of fresh frozen plasma, fibrinogen, or both, as well as on aggressive platelet support to maintain the fibrinogen level above 1.5 g/L (150 mg/dL) and the platelet counts above 30 to 50 × 109/L, until disappearance of all clinical or laboratory signs of coagulopathy. Given the higher risk of developing lethal hemorrhages, these supportive measures should be even more aggressive in older patients, patients with hyperleukocytosis or overt clinical or laboratory signs of coagulopathy, and patients with an abnormally increased level of serum creatinine.28 The benefit of heparin, tranexamic acid, or other anticoagulant or antifibrinolytic therapy to attenuate the hemorrhagic risk remains questionable.
In addition to the supportive measures aimed at counteracting the coagulopathy, physicians caring for patients with APL should be aware of early symptoms or signs suggestive of the so-called APL differentiation syndrome. In fact, patients with APL treated with ATRA as well as those treated with ATO can experience this syndrome. Given the life-threatening nature of the full-blown syndrome, specific treatment with dexamethasone at a dose of 10 mg twice daily by intravenous injection should be promptly started at the very earliest sign or symptom. Temporary discontinuation of ATRA or ATO is indicated only in cases of severe differentiation syndrome. Otherwise, ATRA or ATO should be maintained unless a progression to an overt syndrome or lack of response to dexamethasone is observed. If a favorable response is obtained, dexamethasone should be maintained until symptoms completely disappear, and then ATRA or ATO should be resumed. There is at present no evidence that prophylactic corticosteroid is advantageous in reducing rates of morbidity and mortality associated with this syndrome. Besides these specific measures to reduce the rates of APL differentiation syndrome- and hemorrhage-associated morbidity and mortality, the policy for other supportive measures, including use of hematopoietic growth factors, does not differ from that commonly used for patients with other subtypes of AML. ATO is also associated with prolongation of the QT interval, and careful monitoring is required. Maintenance of the serum potassium and serum magnesium well above the lower limit of normal value is also indicated.18 In addition to the prolongation of the QT/QTc interval, and the APL differentiation syndrome mentioned earlier, approximately 13% of patients develop hypokalemia or hyperglycemia.
Assessment of response after induction therapy
Morphologic, molecular and cytogenetic evaluation should be cautiously interpreted to avoid erroneous therapeutic decisions. Morphologic features in bone marrow during differentiation therapy can lead to erroneously labeling some patients as resistant by inexperienced pathologists. These potentially misleading cytomorphologic features, which are occasionally detectable several weeks after the start of treatment (up to 40–50 days), should not lead to therapeutic changes. Rather, treatment should be continued until terminal differentiation of blasts and achievement of CR, which occurs in virtually all patients with genetically proven APL. As for the early morphologic evaluation, molecular and cytogenetic assessment at the end of induction has little or no value in APL. Clinicians should refrain from making therapeutic decisions on the basis of these results. Thus the assessment of the response should wait until after completion of consolidation therapy.
PostremissionTherapy
Consolidation
The achievement of molecular remission rates of 90% to 99% in patients receiving at least two further cycles of anthracycline-based chemotherapy after induction has led to the adoption of this strategy as the standard for consolidation.18 Currently, there is a tendency to design risk-adapted strategies to modulate treatment intensity during consolidation according to predefined risk of relapse.29 This seems to be an efficient approach to minimize therapy-related morbidity and mortality while maintaining the potential of cure for each relapse-risk group.17 However, some issues related to this phase of therapy remain controversial.
The role of all-trans retinoic acid
The benefit provided by the addition of ATRA to chemotherapy for consolidation has not yet been demonstrated in randomized studies. Nevertheless, historical comparisons of consecutive trials carried out independently by the GIMEMA30 and PETHEMA17 groups showed a statistically significant improvement in outcomes when ATRA at standard dose (45 mg/m2/d for adults; 25 mg/m2/d for children) was given during 15 days in conjunction with chemotherapy, suggesting a synergistic effect of this combination.
The role of cytarabine
From the first successful regimen using daunorubicin in monotherapy2 to the present, the role of cytarabine in APL has remained controversial. None of the studies conducted in the pre-ATRA era, including a randomized one,7 showed an advantage in adding cytarabine to anthracyclines as compared to using high-dose anthracyclines as single agents.5,6 With the incorporation of ATRA into most state-of-the-art regimens, the controversy about the role of cytarabine has remained unresolved. A recent randomized study of the European APL group19 reported an increased risk of relapse when cytarabine was omitted from a schedule including daunorubicin. The conclusions of this interesting study, however, should be interpreted with caution. As a matter of fact, the results of the latter study might have been largely dependent on the particular choice and dose of anthracycline chemotherapy employed (daunorubicin at 495 mg/m2). Of note, a joint analysis of the PETHEMA and the European APL groups31 demonstrated a significantly lower cumulative incidence of relapse in patients younger than 65 years with white blood cell counts less than 10 × 109/L at presentation who were treated with anthracycline monochemotherapy (i.e., with no cytarabine) in the PETHEMA LPA99 trial as compared to patients in the best arm of the European APL 2000 trial including cytarabine. A possible explanation for these discrepancies between chemotherapy regimens with or without cytarabine could be the use of a different anthracycline (idarubicin and mitoxantrone versus daunorubicin). Also, a higher cumulative dosage has been used in the PETHEMA trial. On the other hand, a trend in favor of cytarabine use was observed in the same joint study for high-risk patients. In keeping with this latter observation, a recent study of the GIMEMA group also suggests a benefit in using cytarabine for consolidation in the high-risk group.30
The role of arsenic trioxide
The role of ATO in post-induction therapy for newly diagnosed APL patients is currently being explored not only to consolidate patients who achieve CR with ATO-derived regimens, aiming to minimize or even eliminate chemotherapy,24–27 but also to reinforce standard ATRA plus chemotherapy regimens. This latter approach has been carried out in a large U.S. Intergroup randomized trial, but the results have not yet been reported. Results of the four studies using ATO for induction and postremission therapy are summarized in Table 2 . Overall, these studies show a high antileukemic activity of ATO. However, as for induction therapy, the current recommendation to use ATO for consolidation, aiming to reduce chemotherapy or to reinforce standard ATRA plus chemotherapy regimens, should be restricted to patients included in clinical trials or unfit patients for whom chemotherapy is contraindicated. In developing countries with health care systems that are unable to afford the expenses of the standard therapeutic agents, or where anthracyclines or retinoic acid are not available, ATO produced locally under good quality control at low cost might provide a reasonable and practical alternative in the treatment of APL.8
The role of stem cell transplantation
The role of hematopoietic stem cell transplantation (HSCT) in front-line therapy of APL has changed dramatically during recent years. In fact, the high cure rate obtained using upfront ATRA and chemotherapy indicates that there is no role for HSCT for patients who are in the first molecular remission at the end of consolidation. For the small fraction of patients with persistent minimal residual disease at this point in time, given the poor prognosis of this subset of patients,32 new approaches such as ATO, with or without gemtuzumab ozogamicin, followed by HSCT should be considered. Allogeneic HSCT is the recommended choice for patients with an available HLA-identical donor remaining PCR positive after salvage therapy with ATO, whereas autologous HSCT is a valid alternative for patients ineligible for allogeneic transplant. In the latter case, however, the achievement of PCR-negativity prior to autologous transplantation is considered a mandatory requisite. For the time being, nearly all experience is based on HSCT following ablative conditioning regimens. Data following reduced-intensity conditioning in this disease are currently lacking.
MaintenanceTherapy
Two randomized studies have shown a benefit from administering ATRA maintenance given intermittently11 or continuously.10 However, the continuous schedule for ATRA has been associated with significant toxicity and does not seem to be supported by pharmacokinetic and pharmacodynamic data on this agent.33 The APL93 study of the European APL group11 also showed a lower relapse rate with the triple combination of ATRA, methotrexate, and 6-mercaptopurine that proved particularly effective for patients with an elevated white blood cell count at presentation. In contrast, a similar study of the GIMEMA group,34 reported in abstract form, failed to demonstrate a benefit of maintenance in APL. These apparently contradictory findings suggest that the effectiveness of maintenance would be inversely related to the efficiency of prior therapy. In other words, maintenance therapy would be less useful when induction and consolidation therapy have been more effective. It is conceivable that well-designed studies with quantitative RT-PCR methods assessing minimal residual disease may help to better select patients who would benefit from receiving maintenance therapy.
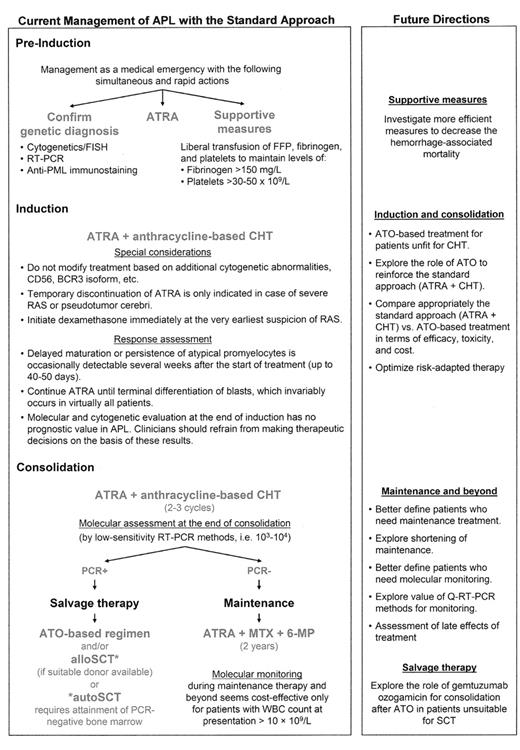
Figure 1 shows the standard approach for the management of APL, including some practical issues, as well as hints of future directions to improve outcomes optimizing the use of the available armamentarium.
Management of Special Situations
APL in older patients
Older patients (60 years or older) are frequently treated with less intensive regimens because of their presumed vulnerability to therapy-related toxicity. The objective should be, however, to offer the appropriate therapy to older patients as well, and only refrain if they are frail or have considerable comorbidities. In fact, they can often be successfully treated even with slightly modified standard approaches with ATRA plus chemotherapy (Table 3 ). Based on the excellent tolerance and high degree of compliance observed in the PETHEMA trials using ATRA and anthracycline monochemotherapy for induction and consolidation therapy,14,17 older patients were treated by this group with the same strategy, dose, and intensity of chemotherapy as used in younger patients, except for a small reduction of idarubicin during induction for patients 70 years or older. This approach provided results comparable with those reported for younger patients, except for the higher mortality rate during remission, especially in patients older than 70 years.35 For those truly frail patients who are considered unfit for chemotherapy, ATO with or without ATRA would be a reasonable alternative to the standard ATRA plus chemotherapy approach.8
APL in children
To our knowledge, only four studies have reported therapeutic results using combinations of ATRA and anthracycline-based chemotherapy (Table 4 ).36 In general, outcome results in children with APL are comparable with those reported in adult patients. To decrease the risk of pseudotumor cerebri during ATRA treatment, a side effect frequently observed in children, some groups have used a reduced dose of ATRA (e.g., 25 mg/m2 instead of 45 mg/m2) for the treatment of children and adolescents with APL. The apparently lower incidence of pseudotumor cerebri and headache, together with the excellent therapy results obtained with ATRA at 25 mg/m2 per day, suggests that this could be the recommended dose, at least for children. Treatment of pseudotumor cerebri consists of temporary discontinuation or dose reduction of ATRA and administration of dexamethasone, osmotic diuretics, and analgesics.
APL in pregnant patients
Management of APL during pregnancy is always a cause for major concern, particularly because of APL-associated coagulopathy and the potential teratogenicity of antileukemic agents. In spite of the limited clinical experience, ATRA and chemotherapy seem reasonably safe when applied to patients with APL during the second or third trimester of pregnancy, as they do not seem to compromise the delivery of a healthy newborn. Stringent fetal monitoring, with particular emphasis on cardiac function, is recommended for patients receiving ATRA during pregnancy because some cases of reversible fetal arrhythmias have been reported. By contrast, although specific information regarding teratogenity of ATRA is lacking, its use during the first trimester of pregnancy should take into account the known teratogenic action of retinoids.
ATO has been shown to be embryotoxic in animal studies. Therefore, although there are no studies in pregnant women, ATO should be avoided throughout pregnancy. Finally, it is also recommended that men and women of childbearing potential use effective contraception, and breastfeeding must be discontinued during treatment with ATO.
Therapy-related APL
A growing number of patients with therapy-related APL (tAPL) have been reported in the last few years. A large multicenter series37 confirmed that tAPL generally develops shortly (< 3 years) after treatment of a primary neoplasm with topoisomerase II–targeted drugs (anthracyclines or mitoxantrone, and less often VP16). Breast carcinoma was by far the most frequent previous tumor (57%), followed by lymphoma, with a large predominance of non-Hodgkin lymphoma compared with Hodgkin disease, whereas other tumor types were found with lower incidence. Patients with tAPL do not seem to have a significantly different prognosis from those with de novo APL when they are managed with state-of-the-art therapy.
Management of CNS relapse
Relapse in the central nervous system (CNS) is uncommon in patients with APL. A joint study by the European APL and PETHEMA groups,38 based on a large series of patients treated with ATRA-based regimens, recently reported a cumulative incidence of CNS involvement at first relapse around 1% after 3 years. This study confirmed the association of CNS relapses with high WBC counts, bcr3 PML-RARα isoform, and age younger than 45 years. However, multivariate analysis only retained high WBC counts. Other presenting features and the occurrence of retinoic acid syndrome were not related to an increased risk of CNS relapse. Although an increased risk of CNS involvement with the use of ATRA and with the omission of high doses of cytarabine has also been suggested, there is no evidence of such associations. Rather, the indisputable increased survival of patients treated with ATRA-based regimens may account for the apparently higher incidence of CNS relapses that otherwise, historically, did not have the opportunity to emerge.
The benefit of CNS prophylaxis has not yet been established. The general consensus is to avoid CNS prophylaxis for patients with white blood cell count lower than 10 × 109/L, in whom the risk of CNS relapse is extremely low. However, because CNS relapses occur in up to 5% of patients with hyperleukocytosis,38 some groups include CNS prophylaxis for patients in this particular high-risk setting. Given that lumbar puncture is extremely hazardous in APL at presentation, it is advisable to postpone CNS prophylaxis for such patients at time of remission.
Salvage Therapy for Relapsed APL Patients
Prior to the demonstration of the striking activity of ATO in relapsed APL, salvage therapy usually consisted of the re-administration of ATRA and chemotherapy for induction, generally containing high-dose cytarabine followed by further chemotherapy and/or HSCT.39 The choice of transplant modality was mainly based on PCR status achieved after chemotherapy: autologous HSCT was the preferred option in patients without detectable minimal residual disease, while allogeneic HSCT was chosen for patients failing to achieve a second molecular remission.40 Currently, given the high antileukemic efficacy observed with ATO in APL patients relapsing after ATRA-containing regimens (Table 5 ), this agent is regarded as the best option in this context. However, the best consolidation strategy after ATO-induced second remission is unknown. Several options are available, including repeated cycles of ATO, combination with standard chemotherapy, and HSCT. In addition, the antiCD33 monoclonal antibody conjugated to caliche-amicin (gemtuzumab ozogamicin) a relatively novel active agent in APL, appears to induce a high rate of molecular responses even as single agent in advanced disease.41,42 Nonetheless, the precise role of this agent in the management of relapsed APL remains unsettled. The selection of one of the above mentioned options, as well as the modality of HSCT, should take into account various variables that may influence the outcome (e.g., molecular status, duration of first remission, age, donor availability).
Current management of acute promyelocytic leukemia and future directions.
Abbreviations: APL, acute promyelocytic leukemia; ATO, arsenic trioxide; ATRA, all-trans retinoic acid; CHT, chemotherapy; FFP, fresh frozen plasma; FISH, fluorescence in situ hybridization; 6-MP, 6-mercaptopurine; MTX, methotrexate; PML, promyelocytic leukemia; RAS, retinoic acid syndrome; RT-PCR, reverse transcription–polymerase chain reaction; SCT, stem cell transplantation.
Current management of acute promyelocytic leukemia and future directions.
Abbreviations: APL, acute promyelocytic leukemia; ATO, arsenic trioxide; ATRA, all-trans retinoic acid; CHT, chemotherapy; FFP, fresh frozen plasma; FISH, fluorescence in situ hybridization; 6-MP, 6-mercaptopurine; MTX, methotrexate; PML, promyelocytic leukemia; RAS, retinoic acid syndrome; RT-PCR, reverse transcription–polymerase chain reaction; SCT, stem cell transplantation.