Abstract
The myeloproliferative disorders have been the “poor cousins” in the family of hematological malignancies for some time. Recently this field has advanced considerably with the description of a mutation in the JAK2 kinase detectable in the majority of patients and the publication of two landmark clinical trials—ECLAP and MRC PT1. But although both ECLAP and MRC PT1 inform clinical management and allude to the complexities of thrombosis we still lack fundamental knowledge, and our understanding of thrombosis in these conditions has not paralleled advances in the field of thrombosis and vascular biology. The predominant clinical complications of essential thrombocythemia and polycythemia vera are thrombotic and hemorrhagic; these significantly impact upon prognosis and quality of life. Here the current status of our knowledge is reviewed with specific emphasis upon the role of the platelet in the pathogenesis of thrombosis as well as the impact of recent data from ECLAP and MRC PT1.
The term myeloproliferative disease (MPD) embraces the conditions chronic myeloid leukemia, polycythemia vera (PV), idiopathic myelofibrosis (IMF), essential thombocythemia (ET) and in the revised World Health Organization (WHO) classification also includes rarer entities such as chronic neutrophilic leukemia. These diseases have in the past been poorly understood. However, the last 18 months have witnessed the identification of a mutation in the pseudokinase domain of JAK2 in a significant number of patients1–4 and the results of two informative clinical studies: the European Collaborative Study of Low dose Aspirin in Polycythemia Vera (ECLAP)5 and Medical Research Council Primary Thrombocythemia 1 (MRC-PT1).6
Hematopoietic progenitors derived from patients with MPDs may be hypersensitive to cytokines such as thrombopoietin or erythropoietin. This has focused research into disease pathogenesis upon downstream receptor events. Several groups concurrently reported an acquired mutation of JAK2 in a majority of patients with PV, as well as almost half of those with ET or IMF.1–4 JAK2 is a member of the Janus kinase family of cytoplasmic tyrosine kinases that are associated with the cytoplasmic domains of cytokine and growth factor receptors. The mutation, located within the negative regulatory pseudo kinase, or Janus homology 2 (JH2) domain, replaces valine with phenylalanine in position 617 (V617F) of the JAK2 protein. To date, correlations with the presence of this mutation and significantly longer duration of disease, a higher rate of complications (fibrosis, hemorrhage, and thrombosis), and treatment with cytoreductive therapy have only been identified by one group.3 Large patient cohorts will be required to fully assess its clinical impact. Potentially JAK2 might highlight patients with differing clinical features as well as differing responses or sensitivity to therapy. Thus JAK2 mutations might guide management in addition to aiding diagnosis and being a potential therapeutic target.
The clinical course of the predominant MPDs (ET, PV, IMF excluding CML) is characterized by thrombotic and hemorrhagic events that significantly impact upon prognosis and quality of life. Thrombosis predominates in ET and PV and these entities are the subject of this article focussing upon the nature of thrombotic events and their pathogenesis, with an emphasis upon platelets. Risk factors for thrombosis and management strategies are briefly discussed.
Thrombosis and Hemorrhage
The precise incidence of thrombosis in PV and ET is hard to ascertain. Confounding factors include the retrospective nature of the literature, comprising relatively small uncontrolled case series, with variable event definition, patient selection and reporting bias. For example, the reported incidence of thrombosis and hemorrhage at diagnosis varies from 12%–39% and 1.7%–20%, respectively, for PV and 11%–25% and 3.6%–37% for ET.7 The majority of hemorrhagic events are minor in nature.
In both ET and PV arterial thromboses dominate venous events. Thrombosis affects large arteries, especially in cerebrovascular and cardiovascular systems for PV. Micro-circulatory disturbances such as erythromelalgia and transient neurological events are by convention characteristic of ET. Erythromelalgia, a microvascular thrombotic syndrome, presents with asymmetric erythema, congestion, and burning pain of hands and/or feet that may progress to acro-cyanotic ischemia and even gangrene. Both ET and PV have an unusual prevalence of intra-abdominal thrombotic events and are the most common (30%–50%) identifiable cause of a thrombosis in this site.8 A thorough evaluation of patients with these thrombotic events to exclude an underlying MPD is indicated but often complicated by difficulties in interpreting laboratory data. The identification of the V617F JAK2 mutation in such patients may prove useful.
Pathogenesis of thrombosis
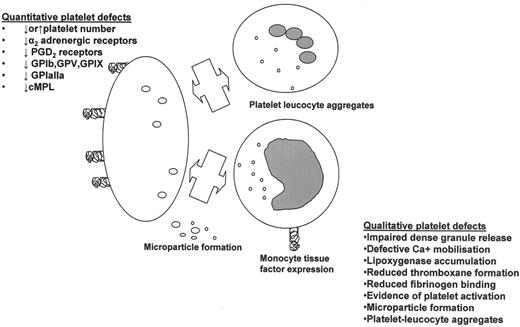
Our understanding of the pathogenesis of thrombosis in MPD has not paralleled advances in the field of thrombosis. Problems limiting research in this area are the prolonged follow up required to generate quality clinical data, numerous confounding clinical factors (e.g., impact of co-morbid conditions, imprecise cytoreductive therapy) and increasingly documented clinical and biological heterogeneity. Multiple factors are likely to contribute to the pathogenesis of thrombosis, including increased cell mass (in PV), possibly platelet number, activation of platelets and leukocytes and their interaction to form platelet leucocyte aggregates, in addition to prothrombotic circulating and endothelial factors. These are each considered in turn with a particular focus upon evidence for a pathogenic role for platelets in thrombosis.
Platelets
The contribution of platelet itself to thrombotic risk is supported by several different lines of evidence. First, histological studies of erythromelalgia demonstrate platelet-rich arteriolar microthrombi rich in vWF and minimal fibrin.9 Second, the exquisite sensitivity of erythromelalgia to aspirin in most patients and, the contribution of platelet count per se as a risk factor has been presumed from clinical data that cytoreductive therapy reduces the incidence of thrombosis.6,10,11 Finally a recently published analysis of the ECLAP data published by Marchioli12 concluded that antiplatelet therapy was significantly associated with a lower risk of cardiovascular events (relative risk [RR] 0.72, 95% CI = 0.53–0.9), but that there was no clear relation between such events and phlebotomy (RR 0.89, 95% CI = 0.67–1.18) or cytoreductive therapy (RR 1.04, 95% CI 0.77–1.41 for HU alone). Whilst this subgroup analysis of ECLAP data implicates the platelet in the pathogenesis of these events any extrapolation of the role of cytoreductive therapy or phlebotomy should be interpreted with caution.
Platelet number
Interestingly, although hemorrhage presumed to be due to acquired von Willebrand disease correlates with the degree of thrombocytosis, there appears to be no direct correlation with thrombosis itself. Nonetheless most available clinical trials in ET and PV suggest that cytoreductive therapy reduces not only the platelet count itself but also the risk of thrombosis. A caveat to this statement being the results from subgroup analysis of ECLAP data (as above12) and the lack of adequate trials comparing hydroxyurea (HU) and phlebotomy in PV. Furthermore, it is not clear whether the benefit of cytoreductive therapy is due to platelet count reduction per se or the overall effect of myelosuppressive agents upon factors including hematocrit (HCT) and white cell count.11 The results of the high-risk arm of MRC PT1 suggest that controlling the platelet count alone in ET patients is not sufficient to protect against most thrombotic complications.6 In that study, 809 high-risk ET patients were randomly allocated low-dose aspirin and either HU or anagrelide. Anagrelide-treated patients were more likely to reach a composite endpoint of major hemorrhage, thrombosis, or vascular death (P = 0.03) despite equivalent long-term control of platelet counts. Arterial thrombosis, major hemorrhage and myelofibrosis were each significantly increased in anagrelide-treated patients (P = 0.004, 0.008 and 0.01, respectively), but venous thrombosis was less frequent (P = 0.006). These data have important implications for our understanding of the pathogenesis of thrombosis as well as choice of therapy for both ET and PV.
Platelet receptors
The hemostatic response of an individual’s platelets is influenced by the quantity and quality of receptors expressed on the platelet surface. A myriad of platelet membrane protein and receptor abnormalities have been reported in MPD including GPIb, GP IIb/IIIa, GPIV, GPVI adrenergic receptors, and the thrombopoietin receptor cMPL. To date however, no consistent correlation between these abnormalities and clinical features of PV and ET have been identified and so the data will not be explored in more detail here.7 Variability in platelet receptors is also determined genetically, and some studies have demonstrated that platelet glycoprotein polymorphisms may contribute to thrombosis. Although the literature contains some conflicting data, even for hematologically normal subjects there is, for example, evidence from a meta-analysis of 12 epidemiological studies of an association between the integrin β3 PLA2 allele and increased risk of coronary heart disease.13 Furthermore reduced platelet sensitivity to aspirin (so-called “aspirin resistance”) may be more prevalent in carriers of PLA2 mutation.14 To date only one study has examined the role of polymorphisms of GPIbα, GPIa, GPIIIa in determining the risk of thrombosis in MPD for patients with PV;15 here the presence of the PLA2 allele of GPIIIa was associated with an increased risk of arterial thrombosis. This data may have physiologic relevance but needs to be confirmed in a larger study. The relevance of polymorphisms of GP1a, GP1bα and integrin α2β1 especially in MPD patients is unclear.
Platelet activation
Enhanced platelet activation in ET/PV was initially documented over a decade ago. More recently, increased expression of P-selectin, thrombospondin and the activated fibrinogen receptor GPIIb/IIIa, amongst others, have been demonstrated and variably correlated with thrombosis. Further features of platelet activation include the formation of platelet microparticles that are associated with the expression with platelet procoagulant activity. One study shows these are increased in ET and PV and correlated with thrombosis.16 These data demand further evaluation both of platelet-derived microparticles and those from other blood cells in MPD patients. Intriguingly, some studies have suggested that cytoreductive therapy may not reverse all abnormal markers of platelet, coagulation pathway and endothelial activation; specifically plasma fibrinogen, P-selectin, von Willebrand factor and soluble vascular cell adhesion molecule (sVCAM).17
Currently, the pathogenesis of platelet activation in MPD is unknown, although a large proportion of patients have a deficiency of lipoxygenase, which could increase availability of endoperoxides to produce thromboxane A2.18 The same patients however have tendency to a hemorrhagic rather than thrombotic diathesis. Alternative explanations for increased platelet activation include an effect of the JAK2-activating mutation, interaction of abnormal HCT, activated white cells, turbulent flow, or an increase in the known priming effect of thrombopoietin19 due to elevated thrombopoietin levels. Recent data suggesting that JAK2 affects cMPL cell surface localization and stability20 may also have implications for the pathogenesis of platelet activation. Activated platelets interact with other blood components both cellular and circulating and have the capacity to provoke endothelial activation/damage.
Platelet interaction with leukocytes and the endothelium
Several authors have measured multiple neutrophil activation parameters in ET/PV. Falanga and colleagues21 measured levels of markers of endothelial damage (thrombo-modulin, VWF antigen) and thrombin activation (thrombin-antithrombin complex, prothrombin fragment 1 + 2, D-dimer) in addition to neutrophil activation (Figure 1 ). Here, enhanced neutrophil activation, evidence of endothelial damage and hypercoagulation were demonstrated in both PV and ET, compared to controls. Endothelial activation may also have been due to elevated levels of vascular endothelial growth factor, which has also been reported to be elevated in ET and PV. Activated leukocytes (neutrophils and monocytes) promote coagulation by release of granule contents, formation of aggregates with platelets. Subsequent effects include platelet leukocyte aggregates playing a pathogenic role in triggering monocyte tissue factor expression22 as well as superoxide anion and inflammatory cytokine release causing endothelial activation and damage.
The interaction between neutrophils and platelets occurs after an adhesion cascade in which CD62P binds to P-selectin glycoprotein ligand-1 on neutrophils. Firm adhesion ensues by binding of CD11b, a β2-integrin in complex with CD18, either to platelet GPIb or to fibrinogen bound to platelet GPIIb/IIIa. Falanga and colleagues have recently extended their previous work by determining in 80 patients (46 ET and 34 PV) compared with 50 controls levels of platelet-neutrophil aggregates, activated neutrophils and platelets and assessed the effect of the addition of f-MLP upon these parameters.23 Neutrophil-platelet aggregates were increased in ET and PV patients and were associated with expression of activation markers CD11b and CD62P. In vitro f-MLP stimulation upregulated CD11b and increased formation of CD11b/CD42b and CD11b/CD62P aggregates without an effect upon platelet activation. Further subanalysis of the ET patients revealed that in those patients taking aspirin alone or in combination with HU the incremental effect of f-MLP upon aggregate formation was significantly attenuated.21 Hence suggesting one of the beneficial effects of aspirin treatment may be to reduce formation of these aggregates. In this study there was no association between increased percentage of neutrophil-platelet aggregates and previous thrombotic events. However, Jensen in 50 patients with MPD reported that those patients with a history of either microvascular or thrombotic events had a greater mean percentage of platelet-leukocyte aggregates.24 These results may offer an alternative mechanism of thrombosis and a possible explanation for the benefit of HU (a pan-myelosuppressive therapy, hence reducing the leukocyte count) when compared to anagrelide in MRC PT1. HU could also protect against thrombosis by its known actions upon gene expression gene expression of endothelin-1 and ICAM-1,25 as well as nitric oxide levels.26
Hematocrit
The hematocrit (HCT) is the major determinant of whole blood viscosity in vitro; however, in vivo flow dynamics and arterial oxygenation also impact upon rheology.27 For example, reduced cerebral blood flow is documented in some patients with increased HCT as consequent increased arterial oxygen content adjusts cerebral flow rate accordingly.27 The HCT also effects platelet activation and the opportunity for platelets to interact with leukocytes and the vessel wall as the axial flow of red cells displaces platelets and plasma to the vessel wall (further affected by the presence of vessel wall disease), thence subjecting them to maximal shear forces. Increasing HCT will naturally result in a narrowed width of the plasma/platelet zone, this has the potential to exaggerate these prothrombotic effects still further.
Risk Factors for Thrombosis
To date few of the pathogenic markers discussed above have been adopted into routine practice to assess an individual patient’s risk for thrombosis (Table 1 ). This reflects limited success in identifying consistently significant factors that can robustly be identified utilizing a simple technique readily applicable to a routine laboratory service. The effect of intercurrent illness, inter-assay variability and MPD-directed therapy upon these tools is also unclear.
In both PV and ET, age and a previous thrombotic event have consistently been proven as independent predictive factors in determining risk of recurrent thrombosis. In the epidemiological aspect of the ECLAP study, age > 60 years combined with a history of prior thrombosis predicted a hazard ratio of 17.3 (P < 0.0001), whereas for prior thrombotic event alone the hazard ratio was 4.85 (P = 0.0099).5 The available data for ET is similar. Cortelazzo described a 3.4% annual per patient risk of thrombosis in those without a previous thrombotic event, compared to 31.4% in those with an event.28
The MPD literature contains conflicting results of the contribution of conventional established risk factors for cardiovascular disease to thrombotic events. In the ECLAP study significant predictors of survival and cardiovascular mortality were smoking, diabetes, congestive cardiac failure, age and prior thrombotic events.5 For the individual patient it is undoubtedly desirable to identify and treat any reversible conventional risk factors for cardiovascular disease. More controversial is the weighting such factors might have in assigning overall risk and hence guiding therapy.7,29 The contribution of common thrombophilic polymorphisms (factor V Leiden, prothrombin G20210A, methylene tetrahydrofolate reductase) to thrombotic risk has not been comprehensively evaluated in MPD. The largest study of 304 ET and PV patients identified an association between presence of the factor V Leiden mutation and venous thrombosis (16%) compared with its absence (3%).30 An increased prevalence of antiphospholipid antibodies has been reported in ET31,32 and correlated with thrombosis in one report.31 Small sample sizes and limited confirmatory testing of the detected antibodies limit the impact of these findings.31 Monoclonal hemopoiesis, reduced expression of c-MPL in megakaryocytes, atypical trephine biopsy features, overexpression of PRV-1 and reduced erythropoietin levels have been associated with thrombosis (reviewed in 33).
Prevention of Thrombosis
The primary rationale for drug therapy for ET and PV is to prevent future thrombohemorrhagic events; whether this prolongs life or influences clonal evolution is unclear. As each therapeutic option is associated with risk of complications, a cornerstone of PV and ET management has been to stratify therapy according to perceived thrombotic risk by utilizing selected risk factors for thrombosis (Table 1 ). In this regard, the definition and therapy of “intermediate risk” patients for ET and PV is particularly controversial with authors variably weighting age, family history, cardiovascular risk factors and thrombophilia. Nonetheless all patients should have assessment and intervention for risk factors for vascular disease, e.g., hyperlipidemia and smoking. Therapeutic options for ET and PV include phlebotomy, aspirin (or an equivalent therapy), with the possible addition of cytoreductive therapy to control myelo-proliferation in high-risk patients (Table 2 ). Cytoreductive drugs to consider for first-line therapy are HU, anagrelide, or interferon-α.
Phlebotomy
In patients with PV the incidence of thrombosis increases from 0.2 episodes/10 years for HCT less than 0.45 to 7.5 episodes/10 years for HCT over 0.60.27 Cerebral blood flow improves by 73% when the hematocrit is less than 0.45;27 thus, phlebotomy to achieve a target HCT < 0.45 is a mainstay of PV treatment. A target HCT < 0.42 has been suggested for females, but evidence is lacking.10
Aspirin
Aspirin has an established role in primary and secondary prevention of vascular disease. Intriguingly, recent data suggest that its primary preventative role may be less in women.34 The ECLAP study found that low-dose aspirin in PV reduced the combined primary endpoint of non-fatal myocardial infarction, stroke, major venous thrombosis or death from cardiovascular cause without a significant increase in hemorrhage in PV.5 Recent subgroup analysis of ECLAP data suggests that aspirin but not phlebotomy or cytoreductive therapy reduced the risk of cardiovascular events (see earlier).12 These data have been translated into management of ET where the evidence for benefit of aspirin is much less substantial. The potential therapeutic roles of the thienopyridines (clopidogrel or ticlopidine) have not been fully studied. However, Ruggeri35 reported no benefit of ticlopidine over aspirin for MPDs with thrombocytosis, and, in fact, microcirculatory events were refractory to ticlopidine. In combination with recent data suggesting that patients with intolerance of aspirin secondary to gastrointestinal effects have a lower incidence of thrombotic events when aspirin was continued with a proton pump inhibitor compared with those switched to clopidogrel36 this finding supports primary use of aspirin.
Hydroxyurea (hydroxycarbamide)
HU has emerged as the treatment of choice for high-risk ET and PV patients because of its proven efficacy. The most controversial aspect of HU therapy is whether or not it is leukemogenic. Biological studies have failed to show an increase in DNA mutations in MPD patients treated with HU.37 Whilst small case series report a variable risk of leukemia after HU, in larger studies the leukemia risk with HU was not statistically different from that in untreated patients.12;29;38 Nonetheless, a randomized trial, such as the on-going Intermediate Risk arm of MRC PT1, will be required to address this question. Clearly, patients who are treated with HU in addition to further cytotoxic agents with leukemogenic potential are at increased risk of leukemia.38
Anagrelide
Anagrelide is approved by the FDA as a first-line agent for control of thrombocytosis associated with an MPD; but only approved for ET patients refractory or intolerant of first-line therapy in Europe. The largest study to date of MPD patients treated with anagrelide (follow-up 7 years) reported reduced disease-related symptoms, no increase in transformation to AML and no data for myelofibrosis.39 The results of MRC PT1 as discussed earlier suggest that HU rather than anagrelide should be first-line cytoreductive therapy for ET and that in any patient the concomitant use of aspirin and anagrelide should be carefully balanced according to the individual risk of thrombosis versus hemorrhage.
Interferon
Interferon-α therapy may satisfactorily control blood counts in more than 75% of patients but it has many side effects and at least 20% of patients may be intolerant. One study reported that interferon-α reduced the rate of venous but not arterial thrombosis.40 This is an interesting parallel to the MRC PT1 data for anagrelide.6
Conclusion
The challenge for the future is to gather high-quality clinical data that can be used to inform decisions about the benefits or risks of therapy. The impact of thrombosis upon prognosis emphasizes the importance of addressing reversible risk factors for these events. The role of the JAK2 mutation is an intriguing and as yet unanswered question.