Abstract
Allogeneic hematopoietic cell transplantation (HCT) has been successfully used as replacement therapy for patients with aplastic anemia and hemoglobinopathies. Both autologous and allogeneic HCT following high-dose chemotherapy can correct manifestations of autoimmune diseases. The impressive allogeneic graft-versus-tumor effects seen in patients given HCT for hematological malignancies have stimulated trials of allogeneic immunotherapy in patients with otherwise refractory metastatic solid tumors. This session will update the status of HCT in the treatment of benign hematological diseases and solid tumors.
In Section I, Dr. Rainer Storb reviews the development of nonmyeloablative conditioning for patients with severe aplastic anemia who have HLA-matched family members. He also describes the results in patients with aplastic anemia given HCT from unrelated donors after failure of responding to immunosuppressive therapy. The importance of leuko-poor and in vitro irradiated blood product transfusions for avoiding graft rejection will be discussed.
In Section II, Dr. Guido Lucarelli reviews the status of marrow transplantation for thalassemia major and updates results obtained in children with class I and class II severity of thalassemia. He also describes results of new protocols for class III patients and efforts to extend HCT to thalassemic patients without HLA-matched family members.
In Section III, Dr. Peter McSweeney reviews the current status of HCT for severe autoimmune diseases. He summarizes the results of autologous HCT for systemic sclerosis, multiple sclerosis, rheumatoid arthritis, and systemic lupus erythematosus, and reviews the status of planned Phase III studies for autologous HCT for these diseases in North America and Europe. He also discusses a possible role of allogeneic HCT in the treatment of these diseases.
In Section IV, Dr. Richard Childs discusses the development and application of nonmyeloablative HCT as allogeneic immunotherapy for treatment-refractory solid tumors. He reviews the results of pilot clinical trials demonstrating graft-versus-solid tumor effects in a variety of metastatic cancers and describes efforts to characterize the immune cell populations mediating these effects, as well as newer methods to target the donor immune system to the tumor.
I. Allogeneic Hematopoietic Cell Transplantation for Aplastic Anemia
Rainer F. Storb, MD
Fred Hutchinson Cancer Research Center, 1100 Fairview North, D1-100, PO Box 19024, Seattle WA 98108-1024
Aplastic anemia is a group of rare disorders that, in their severe form, are characterized by profoundly hypocellular marrow with 2 or 3 of the following peripheral blood cell parameters: neutrophil counts of < 500/μL, platelet counts of < 20,000/μL, and reticulocytes (corrected) of < 1%.1 Approximately 1000 new cases are diagnosed annually in the United States, and they have a high fatality rate without definitive therapy. The age-adjusted case rate in the United States is 2.2 per million population per year.2,3 The rate in European countries is similar to that in the United States while in Korea, Japan, and other Asian countries, the rate is 11 age-adjusted cases per million population per year.2,3
The exact pathogenic mechanisms for aplastic anemia are unknown, and potential mechanisms include intrinsic defects of hematopoietic stem cells, defects in the marrow microenvironment, and abnormal humoral and cellular immune controls of hematopoiesis. Most patients have aplastic anemia of unknown etiology; however, infectious agents, drugs, and chemicals, as well as hereditary causes, have been implicated in the etiology of this disease. Table 1 shows the etiologies among almost 700 patients referred to the Fred Hutchinson Cancer Research Center (FHCRC) through February of 1997.
A very effective treatment for aplastic anemia has been marrow transplantation. The idea of replacing diseased marrow with marrow from a healthy donor goes back to the 1960s with the first successful transplant from a monozygous twin.4 The current chapter will review the results of marrow transplantation for severe aplastic anemia.
Syngeneic Marrow Grafts
Approximately 50% of patients with severe aplastic anemia who received a simple marrow infusion from a monozygous twin without pretransplant conditioning showed complete recovery of their hematopoiesis, consistent with the notion that the disease in these patients was due to a stem cell defect.5– 9 In the remaining half of the patients, second marrow transplants were performed after conditioning with cyclophosphamide (CY), 50 mg/kg on each of 4 successive days. The etiology of aplasia in these patients may be immune or the result of unknown factors whereby the dysfunction of marrow can be overcome by conditioning with CY and a second marrow graft. Overall survivals with twin transplants have been reported to range from 70% to 90%.
Allogeneic Marrow Grafts
A major hurdle that needs to be overcome in the allogeneic transplant setting is the host-versus-graft rejection. This has been accomplished with the help of immunosuppressive conditioning regimens. Studies in rats,10 dogs,11 and rhesus monkeys12 in the 1960s showed that CY, although nonmyeloablative, was a powerful immunosuppressive drug that enabled stable engraftment of transplanted allogeneic hematopoietic cells. Given these properties and the fact that aplastic anemia is a nonmalignant disease characterized by an empty marrow, CY seemed well suited to prepare patients with that disorder for allogeneic marrow grafts. CY was introduced into the clinical practice of marrow grafting in 1969 and has remained a major component of many transplantation protocols (reviewed in13).
HLA-matched related marrow grafts
The largest number of transplants for aplastic anemia performed to date have been from HLA-matched related donors. Major hurdles encountered have been graft rejection, acute graft-versus-host disease (GVHD), chronic GVHD, and late sequelae from conditioning regimens and transfusion support. As the following paragraphs will show, the incidence of graft rejection has decreased, in part due to the use of more effective immunosuppressive conditioning regimens and in part due to changes in transfusion practices. The incidence of acute GVHD has also decreased owing to improved GVHD prevention protocols. Both the incidence and mortality of chronic GVHD also have declined slightly. By avoiding irradiation, late sequelae including impaired growth and development among children and secondary malignancies have decreased. Also, improved screening of transfusion donors has virtually eliminated one major long-term consequence, hepatitis C–associated liver disease. These changes in turn have resulted in significant improvements of survival.
Graft rejection
Graft rejection may occur owing to minor histocompatibility disparities between donor and recipient. Primary rejection is defined by the absence of any signs of hematologic function of the graft, and late rejection as a graft loss after initial graft function. Patients with primary or late graft rejection can often be rescued with second marrow grafts.
The early experience with CY conditioning in aplastic anemia patients showed allograft rejection to be a major problem seen in 35–60% of patients.14–,17 The principal cause of graft rejection has been sensitization of recipients to donor minor histocompatibility antigens by way of preceding blood product transfusions, as shown by extensive animal studies18,19 and confirmed in clinical trials.20 Data in a canine model have shown dendritic cells in the transfusion product to play an important role in sensitizing recipients to minor histocompatibility antigens.21 Exposing blood products to 2000 cGy of gamma irradiation in vitro before transfusion almost eliminated sensitization to minor histocompatibility antigens and prevented rejection of dog leukocyte antigen (DLA)-identical grafts.22,23 Other ways of reducing rejection in the canine model included the use of platelet and red blood cell transfusions that were leukocyte-depleted.24 These data strongly suggested that human patients with aplastic anemia who were candidates for marrow grafting should be managed with irradiated, leukocyte-poor blood products.
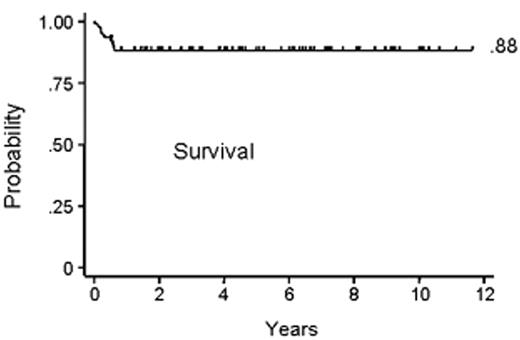
Subsequent canine studies showed a combination of an alkylating agent and antithymocyte globulin (ATG) to be effective in overcoming transfusion-induced sensitization.19,25 Based on these results, a combination of CY, 50 mg/kg/day × 4 days, and horse ATG, 30 mg/kg/day × 3 days, was introduced into clinical transplantation in the late 1970s. First, the regimen was demonstrated to be successful in conditioning patients for second marrow grafts after rejection of their first grafts.26 Encouraged by these results, CY/ATG was used as conditioning for first marrow transplantation beginning in 1988.27Table 2 summarizes the characteristics of aplastic anemia patients conditioned with CY/ATG and given HLA-matched related grafts at 4 transplant centers.28 Median patient age was 26 with a range of 2–59 years. Most patients had received previous transfusions. Approximately one third of the patients had received unsuccessful preceding therapy with immunosuppressive agents. The median duration of aplastic anemia was 2.4 months with a range of 0.2–146 months. Figure 1 shows the survival to be at 88% with a median follow-up of 6 years. Four patients (4%) rejected their marrow grafts (Figure 2A ). Graft rejections occurred between 2 and 7 months after transplant. All 4 patients had received previous blood transfusions. All 4 underwent second transplantations from the same donors, and 3 are alive between 6.5 and 10 years after the initial transplantation. One patient who was not conditioned for second transplantation died from infection 6.5 months after the first transplant and within a day of second transplantation. Chimerism evaluations in surviving patients were carried out between 1 and 6.5 years after transplant and showed a median of 100% donor cells (range 50%–100%).
Other investigators have used conditioning regimens that included CY combined with either whole-body or limited-field irradiation, and these have also resulted in lower rates of graft rejection. Comprehensive recent reports from the International Bone Marrow Transplant Registry (IBMTR) reviewed results in more than 1300 patients transplanted over various time periods (Table 3 ). For the most part, conditioning consisted of CY given either alone or in combination with total body irradiation (TBI) or limited-field irradiation.29 Most recently, several centers have also begun using CY/ATG. During the various time periods beginning in 1976, rejection rates ranged from 11% to 20%. Even though patients were young, acute GVHD in the earlier series was seen in between 37% and 39% of patients. In a more recent series of patients transplanted between 1988 and 1992, the acute GVHD had declined to 19% and survivals ranged from 48% to 66%. The most recent IBMTR report30 described data in patients transplanted between 1991 and 1997 and reported survivals of 75% for patients 20 years and younger, 68% for those between 21 and 40 years, and 35% for those above 40 years.
GVHD
In the early years of marrow transplantation, prevention of acute GVHD consisted of monotherapy with long-term methotrexate (MTX).15 Acute GVHD was seen in 35% of the cases.31 Comparative trials showed MTX and cyclosporine (CSP) to be equivalent in regard to preventing acute GVHD. Both a reduced incidence of acute GVHD and improved survival were seen when a combination of a short course of MTX and CSP was used both in adults and in children.32,33 Results among the 94 patients conditioned with CY/ATG and treated with MTX and CSP after transplantation are summarized in Figure 2B .28 The overall cumulative incidence of grades II–IV acute GVHD was 29%. Specifically, grade II disease was seen in 21%, grade III in 7%, and grade IV in 1% of the patients. The cumulative incidence of chronic GVHD was 32%. In most patients, chronic GVHD responded to therapy with complete responses (Figures 2C and 2D ). Of the patients who developed chronic GVHD, 83% are surviving (Figure 2D). At a median of 2.6 years (range 1.5–10 years) after transplantation, 8 patients still required immunosuppressive therapy for chronic GVHD. The overall incidence of chronic GVHD appeared to be comparable to those in the IBMTR analyses (Table 3 ).
Late sequelae
Neuroendocrine function and growth and development in patients conditioned with CY have been reported to be normal while patients conditioned with irradiation-based regimens, in particular TBI, showed impairment of endocrine functions as well as decreased growth velocity among children.34 Similar findings were made with respect to gonadal function during or after puberty as well as fertility.35 Furthermore, the risks of secondary malignancies appear to be decreased after CY conditioning. Specifically, a report on 330 patients with aplastic anemia conditioned with CY and followed for up to 20 years after transplantation showed a cumulative cancer incidence at 15 years of 3.8%.36 Three quarters of these tumors were carcinomas of skin or oral cavity which are curable by surgery. In contrast, patients conditioned with CY and thoracoabdominal irradiation were reported to have a cumulative cancer incidence of 22% at 8 years.37 Results showed that irradiation-based regimens should be avoided in HLA-matched marrow transplant recipients because of the associated risk of late cancer.38
Grafts from unrelated marrow donors
Generally, the results of marrow grafts from unrelated donors have been less encouraging than those with grafts from HLA-matched siblings. In part, this has been related to the fact that marrow grafts from unrelated donors were carried out only after a failure of one or several courses of immunosuppressive therapy, usually ATG combined with CSP. By the time a marrow transplant was carried out, patients were often infected and refractory to platelet transfusions, complications that adversely influence the outcome of marrow grafts. In part, results of marrow grafts from alternative donors have been worse because of increased risks of both graft rejection and GVHD due to the greater genetic disparity between donors and recipients. Results of several large cooperative studies sponsored by the National Marrow Donor Program (NMDP), the Japanese Marrow Donor Program (JMDP), and retrospective analyses by the IBMTR are shown in Table 4 .30,39– 41 The initial conditioning regimens used varied, ranging from CY and high-dose fractionated TBI to CY combined with various forms of lymph node irradiation (LNI). More recently, an NMDP-sponsored study evaluated CY/ATG combined with de-escalating doses of TBI, from 6 Gy to 2 Gy. Postgrafting immunosuppression also varied, although most frequently the combination of a short course of MTX combined with CSP was used. Graft failure rates ranged from 2% to 11%. The incidence of acute GVHD ranged from 29% to 52%. Chronic GVHD was seen in approximately one-third of patients. Overall survivals ranged from 36% to 58%. Thus, while not quite at the level of the results in HLA-matched related grafts, outcomes of grafts from unrelated donors have steadily improved, even though patients were transplanted only after immunosuppressive therapy had failed. Further improvements are likely with transplants carried out earlier in the course of the patient’s disease, with improved donor selection through state-of-the-art HLA matching, further refinements of the conditioning regimens (a very effective regimen consists of CY/ATG and 2 Gy of TBI), and further improvements in GVHD prevention through better postgrafting immunosuppression.
Conclusions
The improved survival of patients with aplastic anemia who were treated by HLA-matched related marrow transplantation has been, in large part, due to decreased incidences of both graft rejection and acute GVHD. The decline in rejection rates, in turn, resulted from the more judicious use of transfusions before marrow grafting, the removal of sensitizing white blood cells from transfusion products, the decrease in immunogenicity (against minor histocompatibility antigens) of transfusion products by in vitro irradiation, and improvements in the immunosuppressive qualities of the conditioning regimens used to prepare patients for marrow grafting. In regard to the latter, irradiation-based programs have been effective; however, CY/ATG conditioning appeared equally effective. Owing to its lessened immediate and long-term toxicities and the fact that it is nonmyeloablative, CY/ATG appears preferable over irradiation-based regimens. Both incidence and severity of acute GVHD have declined with the introduction of the MTX/CSP regimen, and this also has contributed to improved survival. Chronic GVHD is difficult to treat, and better therapies are needed. One way to guard against further increases in chronic GVHD is to use marrow instead of G-CSF–mobilized peripheral blood mononuclear cell grafts.42 As more and more patients become long-term survivors, the problem of long-term sequelae from the initial conditioning program and from postgrafting immunosuppression must be considered, in particular secondary cancer. Radiation-based regimens have a significantly higher likelihood than CY/ATG of inducing secondary cancer, of slowing growth and development for pediatric patients, and causing sterility. Unrelated grafts are currently considered only for patients who have failed immunosuppressive therapy, and this has adversely influenced outcomes. The least toxic current conditioning regimen for unrelated grafts consists of CY/ATG combined with 2 Gy TBI. Better selection of donors through improved HLA typing, earlier transplantation, and improved GVHD prevention are likely to lead to better outcomes.
II. Stem Cell Transplant in Thalassemia: Allogeneic Gene Therapy
Guido Lucarelli, MD,* Pietro Sodani, MD, Marco Andreani, PhD, Paola Polchi, MD, and Claudio Giardini, MD
Director, International Project on Transplantation in Thalassemia, Hospital of Pesaro, Lombroso Street, 61100 Pesaro, Italy
The cure of thalassemia comes from the correction of the genetic hemopoietic stem cell defect that leads to abnormal globin chain synthesis and thus hemolytic anemia. After the eradication of the thalassemic marrow with the conditioning regimen, the allogeneic normal or heterozygous stem cells infused into the patient are the vectors of a functioning gene that regulates globin chain synthesis. The allogeneic stem cell must overcome the immunological barrier and therefore must be HLA genotypically or phenotypically identical. The dream to use autologous genetically engineered stem cells as the vector of an in vitro corrected gene is at the moment far from clinical application.
We report here the Pesaro experience on bone marrow transplantation in 1003 patients with hemoglobinopathies. The results obtained in subgroups of patients according to the degree of HLA matching, age at the time of transplant, risk class, and protocol used for transplant preparation have been described previously.1,2 The current report focuses on results obtained with ongoing protocols.
Bone marrow transplantation procedure
Prerequisite to transplant for thalassemia is the availability of an HLA-identical donor. The probability of 1 sibling being HLA identical to the patient is 25%, 1 of 2 siblings 43.7%, and 1 of 3 siblings 57.8%. The average probability of having an HLA-identical family member is 30%–36% in thalassemic families in Italy. Once a compatible donor has been identified, the patient is assigned to 1 of the 3 “Pesaro” risk classes. This classification is based on patient characteristics and liver biopsy.2 The transplant protocols for Classes 1 and 2 are the same and based on busulfan, 14 mg/kg total dose, followed by cyclophosphamide, 200 mg/kg total dose. The elaboration of the protocols for Class 3 patients, those patients who have more advanced disease, has required more time and experience. There are two fundamental steps in the pretransplant preparation: eradication of the diseased hemopoietic system by administration of total doses of 14 to 16 mg/kg of busulfan, and suppression of the immune system by administration of a total dose of 200 mg/kg of cyclophosphamide. However, there is an overlap of the antiproliferative activity of busulfan on the immune cell system and of cyclophosphamide on the hemopoietic stem cell system. The combined toxicities of busulfan and cyclophosphamide reach the tolerability threshold, and therefore doses cannot be increased. When the allogeneic graft starts to proliferate in the recipient, an immunological graft-versus-host reaction (which leads to GVHD) may occur, affecting 1 or more organs to varying degrees. GVHD, a serious complication of bone marrow transplantation, can be prevented in most cases by the prophylactic administration of cyclosporine. Three or 4 doses of methotrexate may be given in addition to cyclosporine in the first 15 days after transplant.
Results of the Transplant
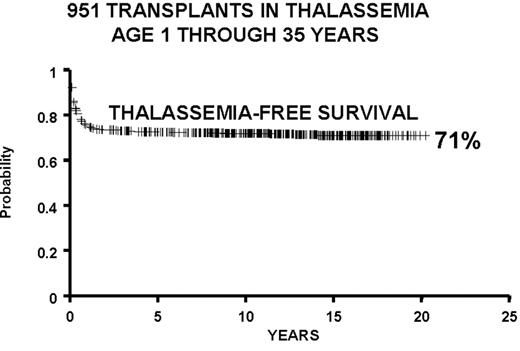
We report the results of a consecutive series of 1003 transplants for patients with thalassemia, ages 1 through 35 years at the time of the transplant, from donors who were either HLA-identical siblings or parents, partially HLA-identical relatives, or HLA-identical unrelated individuals. The series includes 15 second transplants (Figure 3 ). In Figure 4 we report the results obtained in the 951 transplants in thalassemia from HLA-identical siblings or parents, including the 15 second transplants. We also report separately the results of transplant in Class 1 and in Class 2 patients using a preparative regimen unmodified since 1985, without the second transplants (Figure 5 ). Patients aged less than 17 years and included in Class 3, which began in April 1997, received a preparative regimen that was developed to increase eradication of disease marrow and immunosuppression by adding azathioprine, hydroxyurea, and fludarabine to busulfan 14 mg/kg and cyclophosphamide 160 mg/kg. Unpublished data, calculated on the basis of results in 38 patients as of March 2003, show a survival of 94% and a thalassemia-free survival of 80% with a mortality of 7% and a rejection incidence of 14%.
Persistent Donor/Host Mixed Chimerism
Ablation of all of the host hematopoietic cells is necessary to establish conditions for complete marrow engraftment of donor stem cells or complete chimerism. However, we have observed that mixed chimerism is not unusual in our group of transplanted thalassemic patients; it may be transient and evolve into complete engraftment or into graft rejection, or may persist. The latter is present when the coexistence of donor and recipient cells lasts longer than 2 years, producing a “functional graft” with high Hb levels sufficient to correct the genetic defect and to abolish the need for red blood cell transfusions.3 In a group of 335 mostly consecutive patients, all with 2 or more years of posttransplant follow-up, we observed that 108 individuals had mixed chimerism 2 months after transplantation. Thirty-five of the 108 patients rejected their grafts, while none of the remaining 227 patients with complete chimerism rejected their grafts. Among the 227 patients with complete chimerism 2 months after transplant, 4% showed persistent mixed chimerism 2 years after transplant. Perhaps the percentages of host cells early after marrow grafting in these patients were below the limits of our chimerism testing. Thirty-four patients cured of thalassemia (ex-thalassemics) after transplant have remained mixed chimeras for periods of 2–13 years and are transfusion independent with hemoglobin levels ranging from 8.3 g/dL to 14.7 g/dL.
This finding suggests that, while high percentages of recipient cells early after transplant reliably predict rejection, mixed donor/host chimerism present beyond 2 years or later after transplantation may represent a state of mutual tolerance between donor and recipient cells. The reasons why mixed chimerism in some patients is transient, while in others it persists, are still unknown. Perhaps clones of T regulatory cells, which maintain mutual donor/host tolerance, in the latter patients are present.4,5 Further investigation is needed to better understand the mechanisms underlying tolerance in order to develop transplant protocols that would allow us to produce persistent mixed chimerism predictably.
Results of Transplantation from HLA-Matched Unrelated Donors
Thirty thalassemic patients, 14 with Class 1 and 16 with Class 2 disease (median age of 8 years, range 2–20 years) were given transplants from unrelated donors who were matched by high-resolution molecular typing for HLA Class I and Class II alleles. Patients were prepared for transplantation with busulfan, thiotepa, and cyclophosphamide followed by cyclosporine and methotrexate as GVHD prophylaxis. With relatively short follow-up, they showed 96% survival and 85% thalassemia-free survival. Eleven percent rejected their graft with return to the pretransplant conditions. It is likely that bone marrow transplantation from HLA-matched unrelated donors offers results similar to those obtained using HLA-matched family donors.6
Conclusions
The treatment of thalassemias with bone marrow transplantation has become an accepted form of treatment worldwide.7– 20 Perhaps in the future the use of genetically engineered autologous stem cells will allow autologous transplant to cure thalassemia in the majority of thalassemic patients. At present, thalassemic patients with HLA-matched donors who are younger than 17 years should receive allogeneic bone marrow transplantation, given the high cure rate.
III. Hematopoietic Stem Cell Transplantation for Severe Autoimmune Diseases
Peter A. McSweeney, FRACP, FRCPA*
University of Colorado Health Sciences Center, 4200 E Ninth Avenue, B190, Denver CO 80262
Introduction and Background
Autoimmune diseases arise when tolerance to self-antigens is lost and self-directed immune reactions are sufficient to damage host tissues. In experimental models, tolerance to self-antigens may be broken by immunization with self-antigens combined with adjuvants that enhance immune recognition. Human autoimmunity may arise after environmental exposure to foreign antigen leading to development or activation of T and B cells that cross-react against self-peptides. Autoreactive CD4+ T cells appear to be involved in the development of most autoimmune diseases.1 Infectious agents may initiate autoimmunity via molecular mimicry where lymphocytes reacting against foreign antigens attack shared host determinants. Epitope spreading may broaden the autoimmune response.2 Drugs may induce autoimmune disease but are rarely implicated in chronic severe autoimmunity. Other hypotheses for autoimmunity include perturbed regulation of otherwise protective self-reactive lymphocyte populations, development of anti-idiotype reactions and apoptosis induced alterations in self-peptides. Fetal microchimerism also has been implicated as a possible factor in development of autoimmunity.3 Genetic susceptibility to autoimmunity varies among individuals. Concordance for autoimmunity between monozygotic twins exceeds that between dizygotic twins but is well short of complete. Hormonal factors are clearly important because of the preponderance of autoimmune diseases in females. Certain major histocompatibility genes are associated with autoimmunity and other susceptibility genes have been implicated.4 Because genetic factors are overall weakly predictive, environmental exposures combined with genetic susceptibility likely cooperate in generating autoimmune disease.
Autoimmune diseases encompass a broad range of clinical entities with disease manifestations being dependent on the nature of self-antigen(s) involved. Conventional therapies for aggressive autoimmunity have increasingly used intensive immunosuppression such as high-dose methylprednisolone and pulse CY. Application of high-dose immunosuppressive therapy (HDIT) with or without autologous stem cell transplantation (ASCT) can further intensify immunosuppressive treatment. In the mid-1990s pivotal meetings set the stage for HDIT5,6 and guidelines for initial studies were agreed upon. Experimental animal studies and several reports concerning human allografting recipients with coexisting malignancy and autoimmune disease7-9 had provided encouraging preliminary data. For safety reasons, initial emphasis was on testing ASCT despite evidence suggesting that allografting would be more effective. Hypothetically, autoimmune disease might simplistically be viewed as being mediated largely by clonal or oligoclonal populations of self-reactive lymphocytes, with T cells being particularly important.1 Accordingly, active disease might be eliminated or ameliorated if these cells can be destroyed and normal immune function restored. This review will summarize preclinical data and results of larger clinical studies of HDIT and ASCT for autoimmunity. To date, a relatively small number of trials have been published and larger less-detailed analyses have been reported from the registry database established by the European Group for Blood and Marrow Transplantation (EBMT) and the European League Against Rheumatism (EULAR). While the registry studies have significant limitations, they have provided important data as to potential risks and benefits of HDIT. Registry databases show that a wide range of autoimmune diseases has been treated (Table 5 ). Most experience has been with systemic sclerosis (SSc), systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), juvenile idiopathic arthritis (JIA), and multiple sclerosis (MS), on which this review will primarily focus.
Transplants in Animal Models of Autoimmunity
Studies in rodents provided an experimental basis for transplants in autoimmune diseases and extensive reviews are available elsewhere.10-12 Early studies showed that bone marrow transplants could transfer murine SLE to nonaffected animals13 and that allogeneic transplants could prevent disease. These experiments suggested that hematopoietic elements played a critical role in autoimmunity and that disease might be targeted through the hematopoietic system. High-dose cytotoxic therapy with hematopoietic cell transplants from self (autologous or “pseudo-autologous”), syngeneic, or allogeneic donors have been tested in a variety of animal models of autoimmunity including those for SLE, RA, and MS. Cure or prevention of autoimmunity was possible although efficacy varied among models and among stem cell sources. Allogeneic transplants were much more effective than autologous transplants in hereditary or spontaneous models with a strong genetic predisposition to autoimmunity, e.g., the MRL/lpr SLE mouse. In models where autoimmunity was induced with exogenous antigen, e.g., experimental allergic encephalomyelitis or adjuvant arthritis, autografting, and syngeneic transplants were effective. It has been proposed that the latter are more appropriate models for human autoimmunity.12
HDIT and ASCT for Severe Autoimmune disease
Collection of cells for transplant
Investigators have generally chosen to use peripheral blood stem cells (PBSC) except in pediatric studies of JIA where bone marrow has been preferred.14,15 PBSC have usually been collected after mobilization with granulocyte colony stimulating factor (G-CSF) or after priming with CY and then administering G-CSF. G-CSF used alone for PBSC collection has caused increased disease activity in some patients with autoimmune disease,14,16-19 including significant neurological deterioration in patients with MS.16 Prednisone may prevent G-CSF-related MS flares without impairing progenitor cell yields.19 CY-based mobilization may suppress disease, prevent G-CSF-related disease flares, and enhance progenitor cell yields14 but is more toxic and expensive than G-CSF alone and has been associated with fatal outcomes in SSc14 and SLE.20 Efficacy of PBSC mobilization may relate to underlying diagnosis, disease activity, the nature of prior therapy, and the use of CY for mobilization.14 Overall, when G-CSF mobilization can be undertaken with the expectation of adequate progenitor cell yields, it is to be preferred over CY mobilization for cost and safety reasons. However, there may be advantages to either approach based on underlying disease and disease activity; and without definitive comparative studies firm recommendations are difficult.
Graft manipulation
Lymphocyte depletion of autografts has been performed to prevent infusion of auto-reactive cells that may promote return of autoimmunity. By analogy, GVHD, an immunologically-mediated condition occurring after allogeneic transplants, can be prevented by removing T-lymphocytes from hematopoietic cell allografts. In an autologous transplant setting any potential benefits from graft-manipulation may be lost if conditioning therapy does not control the underlying disease and to date autografting studies for malignancy have not demonstrated a convincing role for in vitro tumor purging.
In a study of patients with malignancy and concurrent autoimmunity, a high rate of recurrent autoimmunity was found using unmodified autografts.21 CD34-selection has been the most frequently used technique for lymphocyte depletion due to its commercial availability. Only one prospective randomized study has investigated the value of CD34-selection. In RA patients there was no advantage to CD34 selection over no CD34-selection22 possibly because patients were conditioned with high-dose CY, a regimen associated with a high relapse risk in RA.23 Whether these findings would hold true in the context of more intensive conditioning regimens and/or in other disease settings is uncertain. A potential drawback of high-level graft depletion of lymphocytes is a higher risk of opportunistic infections. While the need for graft manipulation remains unproven, the main underlying hypothesis for these transplants supports continued attempts to eradicate graft lymphocytes through either graft manipulation or in vivo antibody administration. Currently planned Phase III trials (Table 6 ) use one or both of these approaches.
Conditioning Regimens
Conditioning regimens are given to ablate autoimmunity within the recipient, and the infused graft provides rapid hematopoietic recovery and, it is hoped, generates a self-tolerant immune system. The efficacy of different conditioning regimens has not yet been compared in clinical trials. Experimental animal studies showed that more intensive regimens using total body irradiation (TBI) were superior to CY or busulfan alone, likely because of more effective eradication of memory T cells.24 Safety concerns, including those of secondary malignancy, have discouraged some investigators from using more intensive myeloablative regimens that incorporate TBI or busulfan. However, the risks of secondary malignancy as determined primarily in patients with underlying malignancy, do not appear to be increased with TBI used at < 12 Gy as compared to chemotherapy-only regimens.25,26
A variety of regimens have been used for initial trials of HDIT. CY ± antithymocyte globulin (ATG) have been preferred particularly for SLE and RA.27 BEAM (BCNU, etoposide, cytosine arabinoside, melphalan) + ATG or CY/TBI + ATG have been preferred in MS.19,28,29 CY ± TBI ± ATG have been used in SSc and JIA.15,18 Safety concerns together with the presence of disease-related organ dysfunction have in general led investigators to use less intensive regimens than when treating malignancy. ATG or other antibodies frequently have been incorporated into regimens to intensify immunosuppression and provide in vivo graft purging, while not increasing toxicities. However, there are risks from excessive immunosuppression with these antibodies. In 2 patients conditioned with CY/TBI/ATG, substitution of rabbit ATG for equine ATG was associated with profound T-cell depletion that may have precipitated fatal Epstein-Barr virus-related posttransplant lymphoproliferative disorder (PTLD).30 Fatal macrophage activation syndrome has occurred in JIA patients possibly related to T cell depletion.15
Assessing Outcomes After HDIT
Demonstrating disease responses depends on the potential for reversing disease-related abnormalities. Fixed organ deficits such as lung fibrosis in SSc or neuronal degeneration in MS would not be expected to improve whereas dysfunction due to inflammatory lesions, e.g., swollen joints in RA, might be expected to improve. Disease evaluation scales for SSC, SLE, RA, JIA and MS are each different, and studies are best done with multidisciplinary teams that include investigators familiar with performing clinical trials in these diseases. Table 7 summarizes some of these scales. Valid outcome assessments need to include not only survival, response and quality of life, but also the need for continued drug therapy. Follow-up by transplant physicians is necessary to evaluate late toxicities with which disease subspecialists may not be familiar. Relapses of autoimmunity after HDIT may provoke additional immunosuppressive treatment that could promote late toxicities such as infections and myelodysplasia.
Mortality Risks
Transplant-related mortality among patients reported to EBMT was 8.6%.23 There was considerable variation and in early reports mortality was < 10% in MS and RA, and 10%–20% in SLE, SSc and JIA.31 These differences most likely reflect the effects of underlying organ function and prior therapy related to the diseases. Improved patient selection and modified treatment methods may have improved outcomes in recent years.23 Because of the relatively small numbers treated so far, many transplant physicians remain unfamiliar with the unusual challenges these patients present. Outcomes might be expected to be best and to improve most quickly in centers treating the most patients.
Results of Autologous Stem Cell Transplantation for Specific Diseases
Systemic Sclerosis
Diffuse SSc is a rare multisystem disese characterized by generalized, frequently debilitating skin thickening and may affect the lungs, heart, kidneys, and gastrointestinal tract. It is thought to be immunologic in nature with secondary fibroblast involvement and has been treated with immunosuppressive and other agents. Mortality at 5 years is about 40%–50% in patients with high skin scores and cardiac, pulmonary, or renal involvement.32,33 Skin thickening and changes in skin scores have been reported as surrogates for disease severity and survival. The lack of effective therapies together with well-defined prognostic factors for early mortality allowed identification of appropriate candidates for evaluation of HDIT.34
The first major report included 41 patients reported to the EBMT/EULAR registry.35 Patients were treated on multiple protocols and assessments were not standardized. With follow-up at a median of 12 months (range 3–55) improved skin scores (≥ 25% improvement) in 69% of patients and an overall trend toward stable lung functions were found. Survival at 1 year was 73% with 17% dying of treatment complications and 10% of disease.
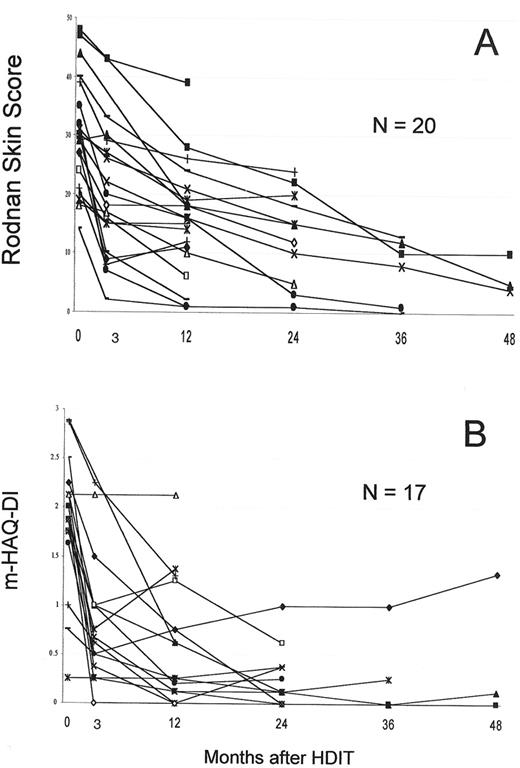
The largest single study was a US collaborative study that included 19 patients.18 PBSC were mobilized with G-CSF and grafts were CD34 selected. HDIT consisted of TBI 8 Gy, CY 120 mg/kg and equine ATG 90 mg/kg. In an initial cohort of 8 patients, 2 died of regimen-related pulmonary injury, a complication avoided in 22 subsequent patients by introducing lung shielding with the TBI. Two other patients died of transplant complications and 2 from progressive SSc. With a median follow-up of 36 (8–65) months, 24 (75%) of 30 patients were surviving. Observations from the first 19 patients showed that among survivors, pulmonary function tests, cardiac function, and renal function were overall stable.18 Major responses were found in 2 important parameters commonly used in clinical trials of SSc. Among 12 patients, the modified Rodnan skin score change at 12 months was -13 (-8 to -13) [P = .0005], with a median decrease of 39%. The mHAQ-DI (range 0–3) changed by a median of -1.675 (0 to 2.26) [P = .002] at 12 months. HDIT produced rapid skin and mHAQ-DI responses of a greater magnitude than in previous SSc trials,36-38 findings confirmed with further follow-up and accrual (Figure 6 ).
A French multicenter study used high-dose CY 200 mg/kg and ATG followed by ASCT with CY-mobilized CD34-selected PBSC.39 Eleven of 12 patients who underwent PBSC collection also underwent HDIT and 8/11 had disease response at some time after transplant. With 18 (1–26) months follow-up, 4/11 (36%) patients had died including 1 from treatment toxicity and 3 from SSc progression. Four additional patients had progressive or nonresponsive SSc requiring further drug therapy between 6 and 12 months posttransplant. The patients in this study had similar baseline characteristics to those in the US study yet disease control and survival appeared inferior.
Systemic lupus erythematosus
SLE is considered the prototypical systemic autoimmune disease because of the spectrum of autoantibodies, diverse clinical manifestations and responsiveness to immunosuppressive agents. Overall survival at 10 years is approximately 90% with early deaths generally related to disease activity and treatment whereas the majority of late deaths relate to atherosclerosis. Intensive CY-based or corticosteroid-based protocols have been effective at suppressing severe SLE, but a small percentage of patients has disease resistant to this therapy.
A study from the Northwestern University group showed that refractory SLE will respond to HDIT.20 CD34-selected grafts were infused after conditioning with CY 200 mg/kg, equine ATG 90 mg/kg and methylprednisolone 3 mg/kg. Of 17 patients, 2 (11%) died after CY-based PBSC mobilization. Among the remaining 15 patients there were no deaths and follow-ups were 2–66 months including 8 with > 24 months follow-up. Patients initially responded well to therapy with reductions in auto-antibody titers, improved SLEDAI scores, improved serum complement levels, reduced prednisone requirements, reduced proteinuria, and in some cases improved serum creatinine. Prednisone was tapered off at 2–18 months post transplant. After initial responses 2 of 7 patients with greater than 30 months follow-up relapsed and required further CY therapy. Data from EBMT included 51 patients with SLE.23 While detailed analyses were not reported, 27 patients improved, 14 improved initially and then relapsed, and 7 died. Overall mortality was 11%, similar to the US study.
Rheumatoid arthritis
RA is the most prevalent autoimmune disease, affecting 1% of the population. Only a small proportion of RA patients have disease resistant to disease modifying antirheumatic drug (DMARD) therapy and severe enough to be considered for HDIT. This population has shrunk since the advent of anti-TNF therapy. Several small pilot studies showed that HDIT could be given safely and induced profound disease responses but that early relapses were common. These observations were confirmed in several larger series. A Dutch study of 14 patients reported significant improvements in disease activity in the first year after HDIT using CY 200 mg/kg and infusion of CY-mobilized CD34-selected PBSC. Four of 12 evaluable patients were nonresponders, and among the remainder all patients relapsed and required DMARDs within 24 months after HDIT.23,40 Synovial biopsy studies showed that T cell infiltrates regressed after HDIT coincident with clinical remissions. In an Australian study, 31 patients were randomized to receive G-CSF mobilized unmodified grafts or CD34-selected grafts after CY 200 mg/kg.22 An ACR20 response was observed in 70%, an ACR50 in 45.4% and an ACR70 in 39.4%. Median time to recurrence was 180 days. There were no treatment-related mortalities and no significant outcome differences between the two arms.
A report from EBMT and ABMTR included 76 patients with a median follow-up of 16 (3–55) months.41 Sixty-six (87%) patients received CY 200 mg/kg ± ATG for conditioning therapy. An ACR50 or better was observed in 67% after HDIT. Significant responses in tender joint counts and HAQ scores were found and these persisted for at least 18 months after HDIT. These sustained improvements were found despite the fact that DMARDs were restarted 73% of patients by 12 months after transplant. There was no treatment mortality indicating that RA patients have a low risk of severe complications after high-dose CY. Overall, these data show that high-dose CY regimens can produce major responses in RA but that these are of relatively short duration. Future investigation of HDIT for RA may focus on improving conditioning therapy and attempting to sustain remissions by adding posttransplant therapies.
Juvenile idiopathic arthritis
A small proportion of children with JIA have disease refractory to intensive conventional therapy with nonsteroidal anti-inflammatory drugs and immunosuppressive agents. Such children can develop severe joint destruction, growth retardation, and complications of long-term immunosuppressive therapy. Core evaluation criteria established for use in clinical trials of JIA are summarized in Table 7 . An initial report of 4 patients suggested efficacy of HDIT42 and a larger study included 31 patients reported to EBMT.15 Inclusion criteria were failure to respond to high-dose methotrexate and at least 2 DMARDs, steroid dependency, and unacceptable treatment toxicity. Bone marrow (n = 23) or PBSC collected after CY + G-CSF mobilization (n = 8) were T cell depleted using T-cell antibodies or CD34 selection. Patients were conditioned with CY 200 mg/kg, ATG 20 mg/kg and, in 21 of the children, with an additional 4 Gy of TBI. Median follow-up after HDIT was 31 (range 8–80) months. Two patients died of macrophage activation syndrome, postulated to be due to T-cell depletion resulting in inadequate control of macrophage activation. Disease recurred in 7 children with mild relapses that were controllable with low-dose prednisone and nonsteroidal anti-inflammatory drugs. Seventeen patients were treatment-free at 8 to 60 months with marked reductions in scores of the CHAQ, physician’s global assessment, and joint swelling.
Multiple sclerosis
Multiple sclerosis has a highly variable course, a long natural history in many patients, and a severe course in relatively few. Major subtypes include relapsing-remitting (RR-MS) disease, secondary progressive (SP-MS) disease, and primary progressive (PP-MS) disease. About 85% of patients have RR-MS at diagnosis and 15% have PP-MS. The extended disability status score (EDSS), a 10-point “nonlinear” scale that measures MS-related neurological dysfunction,43 is used in many clinical trials to determine eligibility and monitor outcomes. MRI studies are also frequently performed in trials to quantify active lesions in the central nervous system. Treatment for RR-MS includes immunosuppressive agents, interferon-beta and glatiramer acetate (Copaxone). Interferon-beta, mitoxantrone, and cladribine may slow SP-MS and no established therapy exists for PP-MS. Unfortunately the magnitude of benefits of these therapies found in large trials is relatively small.
Because initial studies of HDIT in MS were performed primarily to evaluate safety, they included many patients with higher EDSS scores (e.g., 5.0 to 8.0) who may have had low potential for treatment benefits. Nonetheless, MS was stabilized in approximately 70% of patients for periods of up to at least 4 years.19,28,44 In aggregate data from 2 studies using TBI/CY ± ATG, 1 (1.8%) of 53 of patients died of transplant complications,19,29 whereas EBMT reported 5 (6%) transplant-related deaths among 85 patients receiving mainly chemotherapy-only regimens.44
The first major report of 15 patients came from Fassas et al in 1997 and their updated experience with 24 patients was reported in 2000.28 PBSC were mobilized with CY 4 gm/m2 and G- or GM-CSF. BEAM + ATG was used for conditioning and unmodified (n = 15) or CD34-selected cells (n = 9) were used for transplants. Probabilities of progression-free survival (< 1 point increase in EDSS or < 0.5 point for baseline EDSS > 5.0) were 92% for SP-MS (n = 13) at 3 years and 39% for PP-MS (n = 8) at 3.7 years.
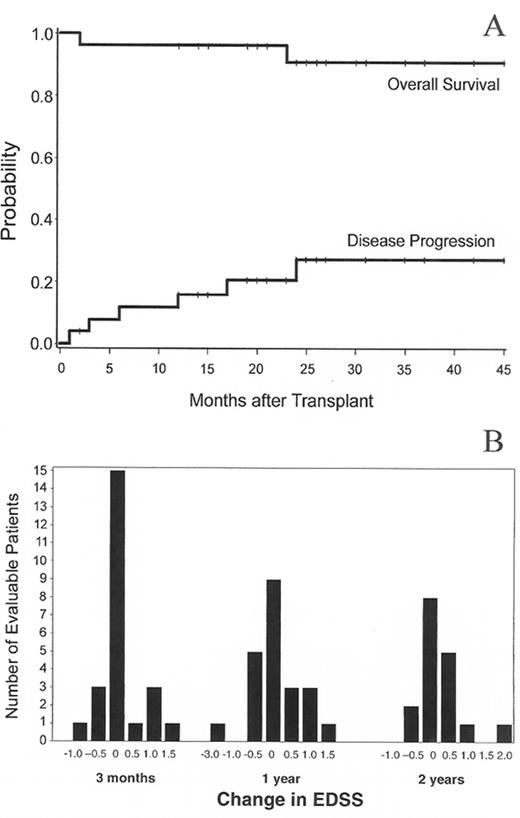
Nash et al reported a multicenter study of 26 MS patients conditioned with TBI 8 Gy, CY 120 mg/kg and equine ATG 90 mg followed by infusion of G-CSF mobilized CD34-selected PBSC.19 Patients had SP-MS (n = 16), PP-MS (n = 8), and RR-MS (n = 1). Median age was 41 (27–60) years and median EDSS 7.0 (5.0–8.0). In some patients neurological deterioration occurred with G-CSF mobilization and with an engraftment syndrome, both of which appeared to be prevented in later patients with prednisone prophylaxis. With a median follow-up of 24 (3–36) months, the 3-year estimates of survival and MS progression were 91% and 27% respectively (Figure 7A ), with stable EDSS scores (< 1.0 point increase) found in 19 of 25 evaluable patients. The distribution of EDSS scores at various time points after HDIT is shown in Figure 7B .
An EBMT registry study included 85 MS patients treated at 20 centers.44 Median age was 39 (20–58) years and median EDSS 6.5 (4.5–8.5). Most patients (86%) had PBSC mobilized with CY + G- or GM-CSF and regimens were BEAM ± ATG (63%), CY + ATG (12%), CY-TBI + ATG (6%), Bu-CY (18%) and fludarabine-ATG (1%). Seven patients died, including 5 from transplant complications and 2 from early neurological progression that may have been treatment-related. At 3 years, progression-free survival (< 1 point EDSS progression) was 74% ± 12% and survival 90% ± 7%. Age > 40 years was an adverse factor and there was a trend for worse outcomes in PP-MS versus RR-MS and SP-MS. Overall, these studies have suggested that HDIT can be applied with reasonable safety in MS. Future studies will test this approach at earlier stages of disease in patients at high risk of rapid progression.
Autoimmune cytopenias
Several small series documented mixed results after attempts to treat autoimmune hemolytic anemia or thrombocytopenia with HDIT and ASCT. The largest report included 14 patients with autoimmune thrombocytopenia (5 had concurrent autoimmune hemolytic anemia–Evans syndrome).45 Six patients experienced durable responses of 9–41 months, 2 partial responses and 6 no response. There were 2 late deaths from catheter sepsis and multiple myeloma.
Phase III Studies of Autologous Transplantation
Despite having relatively limited Phase II data, investigators have initiated Phase III studies in Europe and North America. This is because of the promising initial results observed with HDIT, the relatively slow accrual to date for optimizing transplant approaches in Phase II settings, and the prolonged periods that will be required to complete Phase III studies. Designing control arms for some of these studies has been somewhat difficult because of the lack of established effective therapies for advanced autoimmune diseases. Current and proposed studies are summarized in Table 6 . The SSc studies in Europe and North America were designed in collaboration to match each other closely in terms of eligibility and control arms, with the anticipation that comparison of the respective transplant approaches might be possible after completion of the trials.
High-dose CY without HCT
This approach, which uses CY 200 mg/kg without ASCT, is similar to the use of CY 200 mg/kg with ASCT. Potential advantages include not having to collect progenitor cells and avoiding infusion of autoreactive cells after HDIT. A disadvantage is that the lack of progenitor cell support may lengthen the period of pancytopenia. Based on studies in aplastic anemia, positive outcomes have been reported in SLE,46 autoimmune cytopenias, and myasthenia gravis.
Allogeneic transplants for autoimmune diseases
Data from animal studies and a scattering of small retrospective clinical studies7-9 suggest that allografting would be effective and likely curative of many human autoimmune diseases. If one views aplastic anemia as relevant to other autoimmunity then there is ample evidence for this.47 According to registry databases, a small number of allografts have been performed (Table 5 ), primarily for hematological autoimmunity. While long-term control of RA and Crohn’s disease in patients transplanted for malignancy has been reported,7,9 a relapse of RA48 suggests that disease control will not be invariable. Reduced-intensity allografts provide an opportunity for investigating allografting because of reduced toxicity. However, mortality risks have generally exceeded 10%, a risk that is too high for most patients with severe autoimmune diseases. It is likely that a “graft-versus-autoimmunity” effect from full-donor chimerism would be required for success unless donor immune cells can control autoimmunity in a setting of mixed chimerism.49 The potential importance of donor alloreactivity has been suggested by reports where GVHD induction or donor leukocyte infusion suppressed autoimmunity previously unresponsive to allogeneic transplant.50 Given the hazards of allografting, selected poor-prognosis patients with SSc, SLE, and JIA might be the best candidates for investigational studies.49 Initial experience in a patient transplanted for severe SSc demonstrated sustained complete disappearance of all active disease associated with full-donor chimerism now with 4 years of follow-up (personal communication, R Nash).
Summary and Conclusions
Initial studies have demonstrated some of the promise and shortcomings of HDIT for severe autoimmune diseases. A higher than anticipated relapse rate has highlighted deficiencies in conditioning regimens, graft manipulation, and perhaps our concepts regarding these diseases. It has become clear that treatment approaches need to be tailored to specific diseases and that effectiveness of HDIT will vary among autoimmune diseases. High response rates seen in SLE, SSc, RA, and JIA, and disease stabilization in MS provide optimism that improved survival and quality of life may result from HDIT. Because of this, somewhat of a paradigm shift may have occurred in the field whereby excellent and prolonged responses of severe autoimmune diseases that fall short of a cure may be acceptable provided toxicities of HDIT are not excessive. Furthermore, the idea has emerged that responses from HDIT might be sustained by other therapy given after HDIT. Finally, Phase III studies will evaluate HDIT as compared to conventional therapy in various autoimmune diseases to more fully define the role of this therapy.
IV. Nonmyeloablative Transplantation for Solid Tumors
Richard W. Childs, MD*
Hematology Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, 10 Center Drive, 10/7/c103, MSC 1652, Bethesda MD 20892-1652
The graft-versus-leukemia reaction mediated through transplanted donor immune cells can cure patients with a variety of hematological malignancies.1 While allogeneic hematopoietic cell transplantation (HCT) has become an accepted form of “immunotherapy” for the treatment of hematological malignancies, its ability to induce antitumor effects in nonhematological cancers has only recently been explored. The investigational use of nonmyeloablative HCT in solid tumors is the logical consequence of the increased understanding of the immunological basis for the cure of hematological malignancies following allogeneic transplantation. Although pilot trials are ongoing, graft-versus-tumor effects powerful enough to induce complete or partial remission of some metastatic solid tumors have recently been described.
The Immune System and Solid Tumors
The failure of conventional chemotherapy to improve survival in many metastatic cancers has provided impetus for the development of immune-based treatment approaches for solid tumors. Initial efforts were directed at nonspecific enhancement of innate immunity through the administration of immuno-modulatory cytokines. The regression of metastatic melanoma and renal cell carcinoma (RCC) in 10%–20% of patients receiving interleukin-2 (IL-2) or interferon-alpha has shown that the immune system can be used to complement chemotherapy in the treatment of advanced cancer.2,3 Unfortunately, cytokine therapy is inactive in most solid tumors. Furthermore, it can have significant toxicities when given in high doses. The identification of tumor antigens that induce T-cell mediated tumor rejection in vitro (e.g., MART-1, WT-1, etc) has led to the development of cancer vaccine trials intended to magnify immunity specifically against the tumor.4,5 This remains a dynamic area of investigation that holds promise for the future. At present, however, only a handful of patients have gained clinical benefit from cancer vaccine therapy.
A number of factors limit conventional approaches designed to enhance innate antitumor immunity. Most vaccine trials have used a major histocompatibility complex (MHC) class I restricted peptide-based strategy. As a consequence, immune responses are limited to single antigenic epitopes without an important CD4+ helper T-cell component.7 Even if effective, approaches targeting a single peptide may select for tumor cells lacking the targeted antigen, a process known as “antigen escape.”7 Perhaps an even greater limitation of conventional immunotherapy regimens is that they attempt to enhance a dysfunctional immune system rendered incompetent by prior chemotherapy treatment or long-standing exposure to the immunosuppressive effects of tumors.56
There are a number of reasons to speculate that allogeneic HCT, a procedure that culminates in complete immune replacement, could overcome some of these problems (Table 8 ). First, allogeneic transplantation provides an opportunity to attack the tumor with a chemotherapy-naïve and healthy donor immune system without tolerance to tumor antigens. Second, even if tolerance to the tumor were to develop, infusions of donor lymphocytes could be given to enhance a graft-versus-tumor (GVT) effect.8 Finally, and perhaps most importantly, the repertoire of immune cells capable of recognizing tumor antigens is much greater in the allogeneic setting and potentially includes tumor-reactive T cells that recognize polymorphic variants of minor histocompatibility antigens (mHa).9,10
The Graft-Versus-Tumor Effect
Trials investigating myeloablative allogeneic HCT in patients with chemotherapy-resistant leukemia led to the discovery that graft-versus-leukemia (GVL) or GVT effects occur in a variety of hematological malignancies.1,11 GVT effects are now known to occur not only in acute and chronic leukemias, but also myelodysplastic syndromes, Hodgkin’s and non-Hodgkin’s lymphomas, Epstein-Barr virus related lymphoproliferative disorder (LPD), and multiple myeloma.12,13 The ability of donor lymphocyte infusions (DLI) to induce remission in patients with relapsed chronic myelogenous leukemia (CML) has proven that the GVL effect can be curative.8,14 These observations have recently shifted the focus of allogeneic HCT away from studies exploring “high-dose” conditioning to transplant approaches that capitalize on donor immune-mediated GVT effects. Subsequently, nonmyeloablative transplants were developed to test the hypothesis that that GVT alone could cure some hematological malignancies. Nonmyeloablative regimens are designed to have low toxicity by using well tolerated, highly immunosuppressive conditioning agents that facilitate donor lympho-hematopoietic engraftment. Pilot trials of this transplant approach were first tested in hematologic malignancies with a track record of sensitivity to GVT effects.15–,17 Early clinical results have been encouraging, showing nonmyeloablative conditioning is well tolerated, associated with a high likelihood of engraftment, and a low risk of morbidity and mortality even in high-risk patients.18–,21 Several studies have shown that striking antitumor responses can be induced in a variety of hematological malignancies.22– 24 Importantly, the lower risk of toxicity with this approach has provided a safer transplant modality to test whether GVT effects can be induced in solid tumors.
Graft-versus-Solid Tumor Effects after Allogeneic HCT
GVT effects in hematological malignancies and the regression of disease following treatment with immunomodulators led to the hypothesis that some solid tumors might be susceptible to allogeneic immune attack. As previously discussed, the transplanted donor immune system is able to target mHa that differ between the patient and donor. Hypothetically, solid tumors originating from tissues that are damaged as a consequence of GVHD might express similar mHa, thus making them a target of the donor allo-response. Furthermore, tumor specific antigens potentially could be targeted by donor T cells, in contrast to T cells that have been tolerized to these same antigens in the patient. However, one can only speculate which tumors would be most susceptible to a GVT effect since the factors dictating the wide variability in susceptibility of hematological malignancies to GVL effects are not well understood.
The first evidence supporting the existence of a graft-versus–solid tumor effect came from murine studies showing a reduction in the incidence of spontaneously occurring lympho-sarcoma in NZ B/W hybrid mice receiving an allogeneic bone marrow transplant.25 Subsequent experiments demonstrated that antitumor effects could be generated against murine mammary carcinoma cells after allogeneic but not autologous bone marrow transplantation.26,27 These murine experiments provide preliminary evidence that mHa that are expressed on tumor cells and in the allogeneic transplant setting are potential targets for a GVT effect.
Some of the first reports of graft-versus–solid tumor effects in humans came from anecdotal observations of metastatic breast cancer regressing concomitantly with GVHD in recipients of a myeloablative HCT.28,29 Subsequently, these observations were confirmed in a small pilot trial of HCT for metastatic breast carcinoma.30 Unfortunately, enthusiasm for these observations was tempered by the significant morbidity related to myeloablative therapy.
Nonmyeloablative HCT as a Platform to Test for GVT Effects in Solid Tumors
Trial design
Without evidence to support its efficacy, and with a 25% or higher risk of transplant-related mortality, many considered experimental studies of myeloablative allogeneic HCT in solid tumors too risky. The preliminary success and improved safety of nonmyeloablative transplant regimens provided the basis for applying allogeneic immunotherapy to patients with treatment-refractory solid tumors. Anticipating a lower risk of transplant-related mortality, studies of nonmyeloablative HCT in patients with metastatic solid tumors were initiated in the late 1990s. Given their investigational nature, pilot trials have been limited to patients with treatment-refractory metastatic disease who have HLA identical siblings to serve as donors. Most trials have been designed with the same two general goals of (1) minimizing toxicity through the use of reduced intensity conditioning and (2) optimizing the induction of a GVT effect by the early withdrawal of GVHD prophylaxis and administration of posttransplant donor lymphocyte infusions and/or immuno-modulatory cytokines (Figure 8 ). Patient and tumor characteristics hypothesized to be optimal for investigational allogeneic immunotherapy trials are shown in Table 9 .
Preliminary clinical results
Renal Cell Carcinoma (RCC): Regression of treatment-refractory metastatic solid tumors following nonmyeloablative HCT has recently been described in a number of different tumors. We chose to first test for GVT effects in metastatic melanoma and RCC because these tumors are considered “immuno-responsive.” Furthermore, both are resistant to chemotherapy, and once metastatic, offer virtually no chance for long-term survival. Patients received conditioning with cyclophosphamide (60 mg/kg x 2 days) and fludarabine (25 mg/m2 x 5 days), followed by infusion of unmanipulated, G-CSF mobilized peripheral blood hematopoetic cell grafts from 6/6 or 5/6 HLA-matched sibling donors. Decisions regarding the timing of GVHD prophylaxis withdrawal were based on disease status and T-cell chimerism.18 Patients with stable disease and full donor T cell chimerism on day 30 initiated a 6-week cyclosporine taper beginning 2 months after the transplant. In contrast, those with mixed T-cell chimerism or disease progression had their cyclosporine withdrawn earlier.
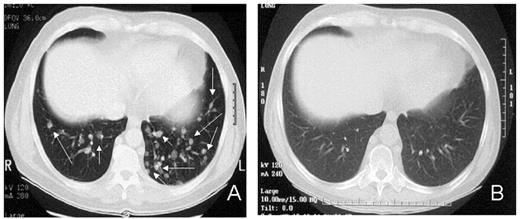
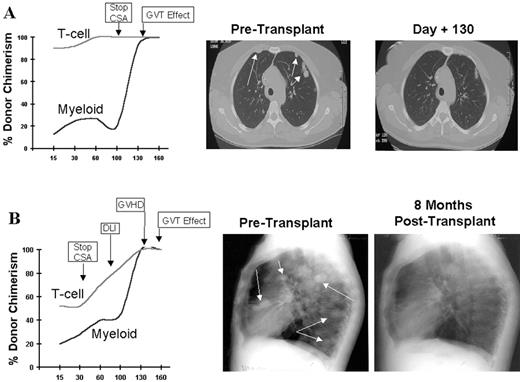
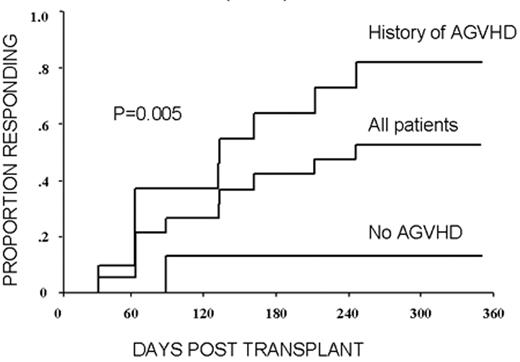
RCC was quickly identified as a target for a GVT effect.31 Ten of the first 19 patients had disease responses including 7 partial and 3 complete responses.32 The first patient treated remains without evidence of disease 5½ years after transplantation (Figure 9 ). Remarkably, all responding patients had failed prior treatment with cytokine-based therapy. Disease responses occurred mostly commonly in patients with pulmonary-restricted metastatic disease, although regression of bulky tumors in multiple metastatic locations has occasionally been observed. Disease responses were typically delayed, occurring 4 or more months after the transplant only after T-cell chimerism had converted from mixed to full donor in origin. Representative lineage, specific engraftment profiles, and their relationship to clinical outcome are shown in 2 patients who underwent nonmyeloablative transplantation and had graft-versus-RCC effects (Figure 10 ). Although GVHD was favorably associated with a disease response (Figure 11 ), some patients had dramatic regression of metastatic disease without having acute or chronic GVHD. Disease progression in the first few months after the transplant did not necessarily preclude a GVT effect; a unique pattern of tumor growth followed by subsequent disease stabilization or regression was observed in several patients. Furthermore, some patients who did not respond to cyclosporine withdrawal had tumor shrinkage after a donor lymphocyte infusion or following treatment with low-dose subcutaneous interferon-alpha.
Several groups have recently reported GVT effects in metastatic RCC using a variety of different conditioning approaches (Table 10 ).33–,36 Although these trials have had small patient numbers, the observation of disease regression in cytokine-refractory patients, including those who had previously failed high dose IL-2, highlights the powerful nature of the GVT effect in this tumor. It is clear from all these studies that careful patient selection is a prerequisite for transplantation, as patients with rapidly progressing metastatic disease are likely to succumb to disease progression before a GVT effect can occur.37
Advances in understanding the tumor targets and effector populations responsible for GVT effects in RCC are necessary for the development of more effective transplant approaches. Disease regression associated with cyclosporine withdrawal, full donor T cell chimerism, and GVHD all provide strong indirect evidence that donor T cells play an important role in these responses. The observation that tumor regression can occur with or without acute GVHD suggests that both broadly expressed minor histocompatibility antigens, as well as antigens that are restricted to the tumor, are potential targets for allogeneic immune effectors. Preliminary in vitro data have shown that RCC cells express a broad range of mHa that could render them susceptible to a GVT effect in the setting of GVHD.38,39 Along these lines, T-cell clones that kill both patient RCC and hematopoetic cells have been isolated from responding patients.40,41 Furthermore, in a few patients without GVHD, MHC class I restricted T-cell lines with tumor-specific cytotoxicity have been expanded in vitro from the patient’s blood at the time of tumor regression (Figure 12; see Appendix, page 605). Studies to identify potential RCC-restricted antigens using these allogeneic T-cell populations are being pursued. These preliminary findings suggest that distinct T cell populations recognizing tumor restricted antigens and/or antigens shared by both the tumor and normal tissues (i.e., mHa) may be involved in these GVT effects.
Metastatic melanoma: While some patients with metastatic RCC have clearly benefited from allogeneic immunotherapy, the results of nonmyeloablative HCT in patients with metastatic melanoma have thus far been disappointing.37,42,43 Anecdotal reports of metastatic disease progressing at “explosive” rates after allogeneic HCT are particularly distressing. In an analysis of 25 patients with metastatic melanoma undergoing nonmyeloablative HCT at 4 transplant centers, median survival was only 100 days with rapid disease progression being the major cause of death.43 Several patients were observed to have tumor regression in the immediate posttransplant period, although these responses were transient and almost certainly the consequence of chemotherapy. One patient had delayed regression of subcutaneous metastasis consistent with a GVT effect; unfortunately, death from CNS metastasis occurred shortly thereafter. The demonstration of clinically beneficial GVT effects in RCC make these results at first seem perplexing. However, they highlight potential limitations of nonmyeloablative HCT in treating solid tumors, where rapid growth kinetics associated with short survival may preclude the opportunity for a delayed GVT effect.37
Other solid tumors: The observation of GVT effects in patients with RCC has led to a rise in the number of investigational transplants being conducted for other types of metastatic solid tumors. However, at this time, insufficient data are available to comment whether GVT effects can occur in most solid tumors. The disappointing results of autologous transplantation in breast cancer have inspired studies of nonmyeloablative allogeneic transplantation in this malignancy. GVT effects, often in association with GVHD, have recently been described in a number of small case series and abstracts.34,44 Investigators from Milan reported 2 delayed GVT effects among 6 women with metastatic breast cancer receiving a thiotepa/fludarabine-based transplant approach. In both cases, responses occurred after a donor lymphocyte infusion.34 Furthermore, unpublished retrospective data from the International Bone Marrow Transplant Registry suggest the risk of relapse/progression may be improved in breast cancer patients who develop GVHD after nonmyeloablative HCT.
GVT effects have also been described in treatment resistant ovarian cancer.45,46 A GVT effect was suggested from a case report of a patient with treatment resistant ovarian cancer undergoing a myeloablative HCT who had a delayed normalization in CA-125 levels associated with a durable (> 2 year) complete response.47 Subsequently, 2 of 4 patients with metastatic ovarian cancer were reported to have disease regression after a buslphan-based nonmyeloablative transplant, although the proximity of these responses to the conditioning regimen makes it difficult to conclude with certainty that disease regression occurred as the consequence of an immune effect.48
Anecdotal reports of GVT effects occurring after allogeneic HCT have also recently been described in patients with metastatic adeno-carcinoma of the colon, pancreas, squamous cell lung carcinoma, neuroblastoma, or refractory sarcomas.35,49– 52 Results from larger case series in these malignancies will likely be forthcoming. Because even low-dose chemotherapy can occasionally have surprising activity in many solid tumors, sequential radiographic imaging studies are required to help discern whether tumor regression is related to the conditioning regimen or the consequence of a GVT effect.
Future Directions
Nonmyeloablative allogeneic HCT trials showing GVT effects in metastatic solid tumors have provided further proof of the strength of allogeneic immunotherapy. Studies evaluating nonmyeloablative approaches using unrelated donors for RCC have recently been initiated, and if effective, could expand the application of allogeneic immunotherapy to a far greater number of patients. Whether GVT effects might be more effective in patients with minimal residual disease, such as after autologous transplantation or aggressive surgical debulking, is also being explored. However, while little doubt exists about its potential application, the use of allogeneic HCT to treat solid tumors will likely remain limited until both the safety and efficacy of the approach are improved (Table 11 ). Concerns over transplant-associated complications continue to make referring oncologists reluctant to send patients for these investigational studies. As a consequence, the approach is further limited by its obligate enrollment of patients with advanced, treatment refractory disease, often with life expectancies far too short to benefit from a delayed GVT effect.
Further progress in the field will require the development of strategies that limit GVHD while targeting the allo-response to the tumor. Murine models have already demonstrated that tumor-specific immunity can be boosted by posttransplant tumor immunization.53 A similar strategy incorporating posttransplant vaccination or the adoptive infusion of tumor specific donor lymphocytes could be used in humans to enhance the efficacy of the procedure.
Another promising approach would be to exploit the ability of natural killer (NK) cells to mediate antitumor responses in the setting of HLA-mismatched transplantation. NK cells are known to induce GVL effects in patients with acute myelogenous leukemia (AML) receiving transplants from mismatched donors when killer immunoglobulin-like receptor (KIR) ligand incompatibility exists in the graft-versus-host direction.54 We have recently shown that NK lines generated from KIR mismatched allogeneic donors can kill both melanoma and RCC tumor cells in vitro, specifically through KIR/KIR ligand incompatibility.55 These preliminary data suggest the antitumor effects seen against AML following KIR incompatible allo-transplantation might also occur in a similar setting against select solid tumors. Refinements such as these hold promise that allogeneic immunotherapy will be used more successfully in the future. For now, however, nonmyeloablative allogeneic HCT is a promising approach for some solid tumors that should remain restricted to investigational trials.
Kaplan-Meier curve of survival among the 94 patients who underwent transplantation. The event-free survival rate was 85% (data not shown).
Reprinted with permission from
Kaplan-Meier curve of survival among the 94 patients who underwent transplantation. The event-free survival rate was 85% (data not shown).
Reprinted with permission from
Cumulative incidences of (A) graft rejection, (B) developing acute grade II–IV GVHD in patients with aplastic anemia given HLA-identical marrow grafts following CY/ATG and GVHD prophylaxis with MTX/CSP, (C) prevalence of chronic GVHD, and (D) probability of survival among the 29 patients with chronic GVHD and probability of discontinuing immunosuppression given for chronic GVHD.
Reprinted with permission from
Cumulative incidences of (A) graft rejection, (B) developing acute grade II–IV GVHD in patients with aplastic anemia given HLA-identical marrow grafts following CY/ATG and GVHD prophylaxis with MTX/CSP, (C) prevalence of chronic GVHD, and (D) probability of survival among the 29 patients with chronic GVHD and probability of discontinuing immunosuppression given for chronic GVHD.
Reprinted with permission from
All protocols used in 1003 consecutive transplants in thalassemia from December 17, 1981, through January 30, 2003, and calculated as of March 1, 2003: 42 partially matched, 15 second transplants, 11 HLA-identical matched unrelated donors.
All protocols used in 1003 consecutive transplants in thalassemia from December 17, 1981, through January 30, 2003, and calculated as of March 1, 2003: 42 partially matched, 15 second transplants, 11 HLA-identical matched unrelated donors.
All protocols used in 951 consecutive transplants from HLA-identical donors in thalassemia from December 17, 1981, through January 30, 2003, and calculated as of March 1, 2003: 15 second transplants and 11 HLA-identical matched unrelated donors.
All protocols used in 951 consecutive transplants from HLA-identical donors in thalassemia from December 17, 1981, through January 30, 2003, and calculated as of March 1, 2003: 15 second transplants and 11 HLA-identical matched unrelated donors.
Patients younger than 17 years old at the time of the transplant who received bone marrow from HLA-identical siblings or parents, from June 20, 1985, through January 30, 2003, and calculated as of March 1, 2003.
Abbreviations: BU, busulfan; CY, cyclophosphamide.
Patients younger than 17 years old at the time of the transplant who received bone marrow from HLA-identical siblings or parents, from June 20, 1985, through January 30, 2003, and calculated as of March 1, 2003.
Abbreviations: BU, busulfan; CY, cyclophosphamide.
Responses in systemic sclerosis (SSc) patients with follow-up of > 1 year.
A. The modified Rodnan skin score which measures skin thickness at multiple sites by palpation and has a range of 0–51.
B. The mHAQ-DI, a functional index with a scale of 0–3.
Responses in systemic sclerosis (SSc) patients with follow-up of > 1 year.
A. The modified Rodnan skin score which measures skin thickness at multiple sites by palpation and has a range of 0–51.
B. The mHAQ-DI, a functional index with a scale of 0–3.
High-dose immunosuppressive therapy (HDIT) for multiple sclerosis (MS).
A. Survival and MS progression after HDIT. Estimated survival was 91% and MS progression was 27% at 3 years. B. Distribution of changes in Extended Disability Status Scale (EDSS) scores at 3, 12, and 24 months after HDIT as compared to baseline.
From Nash RA, Bowen JD, McSweeney PA, et al. High-dose immunosuppressive therapy and autologous peripheral blood stem cell transplantation for severe multiple sclerosis. Blood. In press. Copyright American Society of Hematology, used with permission.
High-dose immunosuppressive therapy (HDIT) for multiple sclerosis (MS).
A. Survival and MS progression after HDIT. Estimated survival was 91% and MS progression was 27% at 3 years. B. Distribution of changes in Extended Disability Status Scale (EDSS) scores at 3, 12, and 24 months after HDIT as compared to baseline.
From Nash RA, Bowen JD, McSweeney PA, et al. High-dose immunosuppressive therapy and autologous peripheral blood stem cell transplantation for severe multiple sclerosis. Blood. In press. Copyright American Society of Hematology, used with permission.
Strategies to enhance graft-versus-tumor (GVT) effects in solid tumors after nonmyeloablative hematopoietic cell transplantation (HCT).Abbreviations: DLI, donor lymphocyte infusion; IL-2, interleukin-2; GVHD, graft-versus-host disease.
Strategies to enhance graft-versus-tumor (GVT) effects in solid tumors after nonmyeloablative hematopoietic cell transplantation (HCT).Abbreviations: DLI, donor lymphocyte infusion; IL-2, interleukin-2; GVHD, graft-versus-host disease.
Delayed regression of metastatic renal cell carcinoma (RCC) following a nonmyeloablative transplant.
A) Extensive metastatic renal cell carcinoma (clear cell histology) involving the lungs had grown slightly 1 month after a nonmyeloablative transplant. B) Complete regression of metastatic disease following cyclosporine withdrawal 4 months after transplantation consistent with a graft-versus-tumor (GVT) effect. Disease remission is ongoing more than 5 years after the transplant.
Delayed regression of metastatic renal cell carcinoma (RCC) following a nonmyeloablative transplant.
A) Extensive metastatic renal cell carcinoma (clear cell histology) involving the lungs had grown slightly 1 month after a nonmyeloablative transplant. B) Complete regression of metastatic disease following cyclosporine withdrawal 4 months after transplantation consistent with a graft-versus-tumor (GVT) effect. Disease remission is ongoing more than 5 years after the transplant.
Engraftment profiles and clinical outcome in two renal cell carcinoma (RCC) patients with a delayed graft-versus-tumor (GVT) effect after nonmyeloablative transplantation.
Patient A had a delayed GVT effect in the absence of acute or chronic graft-versus-host disease (GVHD), while patient B had disease regression following a donor lymphocyte infusion (DLI) in association with acute GVHD.
Abbreviations: CSA, cyclosporine.
Engraftment profiles and clinical outcome in two renal cell carcinoma (RCC) patients with a delayed graft-versus-tumor (GVT) effect after nonmyeloablative transplantation.
Patient A had a delayed GVT effect in the absence of acute or chronic graft-versus-host disease (GVHD), while patient B had disease regression following a donor lymphocyte infusion (DLI) in association with acute GVHD.
Abbreviations: CSA, cyclosporine.
Association of acute graft-versus-host disease (AGVHD) with a disease response in renal cell carcinoma (RCC) (n= 19).
Reprinted with permission from
Association of acute graft-versus-host disease (AGVHD) with a disease response in renal cell carcinoma (RCC) (n= 19).
Reprinted with permission from
Grant support: CA78902, CA18029, CA15704, CA18221, HL36444 from the National Institutes of Health, Bethesda, MD