A recent Nature article by Okashita et al1 demonstrates that severe maternal iron deficiency can cause male-to-female sex reversal in mouse embryos. Their novel and unexpected finding highlights the contribution of epigenetic regulation to mammalian sex determination and underscores iron’s widespread biological importance and fundamental role in development.
Iron is thought to have catalyzed the evolution of earliest life forms, so it is not surprising that iron remains essential for fundamental biological processes. These include enzymatic reactions that govern DNA and histone demethylation, and thus, epigenetic control of gene expression. Many demethylases require iron for their activity; specifically, ten-eleven translocation (TET) DNA demethylases and JmjC-domain–containing histone demethylases (JHDMs) require iron as a cofactor. These demethylases have been shown to influence epigenetic control of cell fates in different organs: B-cell proliferation,2 adipocyte differentiation,3 and fetal brain development.4 If cellular iron was low enough to impair the enzymatic activity of demethylases, it could exert profound epigenetic effects and alter cell fate determination.
Sex determination in mammals is driven largely by the sex-determining region Y gene (SRY) located on the Y chromosome (reviewed in Wilhelm et al5). SRY encodes a transcription factor whose primary role is to trigger the development of testes from undifferentiated bipotential gonads that all embryos initially possess. In mice, Sry expression in gonads is tightly regulated in a spatiotemporal manner. Its expression is selectively induced in pre-Sertoli cells (the first cells in the male gonad to undergo sex-specific differentiation) starting at embryonic day 10.5 (E10.5), peaking at E11.5, and waning by E12.5.6 Disruption of Sry expression during this time frame, be it genetic or epigenetic, can cause primordial gonads of XY mice to aberrantly differentiate into ovaries.7
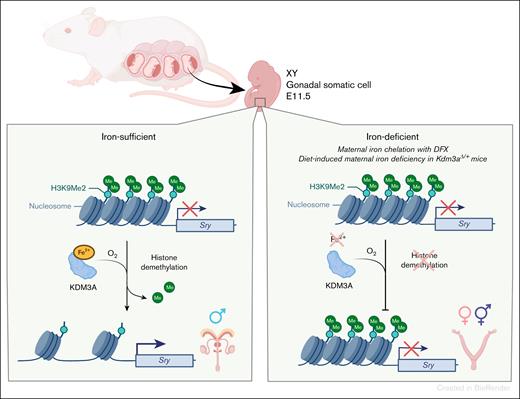
Previous work from this group demonstrated the critical importance of Sry epigenetic regulation to sex determination.8 The Sry locus is transcriptionally repressed in most cells, including in cells of the early embryo by H3K9me2 (histone H3 which is dimethylated at its ninth lysine residue). Demethylation of this histone by demethylase KDM3A (JHDM family) in pre-Sertoli cells initiates male sex determination.
The current study links iron to the epigenetic determination of male sex in mammals using multiple complementary and highly technical approaches.1 They first demonstrated that pre-Sertoli cells initiate iron acquisition phenotype by increasing cellular iron uptake and decreasing iron export. Compared to other gonadal somatic cells, they had higher expression of the iron uptake genes Tfrc and Scara5 and decreased expression of the iron export genes Slc40a1 (Fpn) and Heph. Disruption of iron uptake by selective deletion of Tfrc in gonadal somatic cells before sex determination reduced cellular iron content by ∼50% and resulted in increased gonadal H3K9me2, accompanied by decreased expression of Sry mRNA. Consistent with their previous work, higher H3K9me2 and lower Sry mRNA resulted in male-to-female sex reversal (as determined by morphology of external/internal genitalia) in 18% (7/39) of animals. These results connect a reduction in gonadal cellular iron to male-to-female sex reversal. Similarly, in an in vitro gonad culture system, iron chelation with deferoxamine (DFO) prior to gonad differentiation increased levels of H3K9me2 at the Sry promoter and decreased expression of Sry mRNA, skewing 90% of XY gonads toward an ovarian signature. Transgenic expression of Sry or restoration of KDM3A-mediated H3K9 demethylation in DFO-treated XY gonads restored the male phenotype, confirming that the effect of iron deficiency was KDM3A- and SRY-dependent. Iron chelation resulted in downregulation of ∼40% of KDM3A target genes, suggesting that iron deficiency during gonadal development compromises KDM3A demethylase activity.
To assess the pathophysiological relevance of this mechanism, the researchers investigated whether maternal iron deficiency during pregnancy was sufficient to impact sex determination in mice. First, they induced maternal iron deficiency with oral iron chelator deferasirox (DFX) administered to pregnant mice daily from E6.5 to E10.5, the time prior to maximal Sry expression in pre-Sertoli cells. E11.5 offspring were expectedly iron depleted and anemic, with decreased gonadal Sry expression. At E13.5, XY gonads had mixed cell composition (“ovotestes”), with ∼20% of cells displaying ovarian cell signature. After birth, of 72 XY mice born to DFX-treated dams, 7% of exhibited aberrant sex organ development. Although these studies raise concerns about therapeutic use of iron chelators in human pregnancies, already the current practice is to discontinue these drugs during pregnancy because of long-standing concerns about their teratogenicity.
To assess the effect of dietary iron deficiency, dams were placed on a low-iron diet for 6 weeks (4 weeks prior to pregnancy and during 2 weeks of pregnancy). Despite severe anemia (dam Hgb ∼7 g/dL vs 12 g/dL), and increased Tfrc expression in E11.5 pre-Sertoli cells in the iron-deficient group, none of the XY embryos exhibited sex reversal (n = 58). However, when diet-induced maternal iron deficiency was induced in haploinsufficient Kdm3a mice, which are predisposed to impaired H3K9 demethylation, ∼5% of XY offspring (2/43) exhibited male-to-female sex reversal. Thus, although diet-induced maternal iron deficiency alone is insufficient to influence sex-determination in mice, iron deficiency may be a modifier in settings which predispose cells to ineffective epigenetic regulation. The proposed mechanism is summarized in the figure.
Iron deficiency can cause male-to-female embryo sex reversal. Testis formation is initiated by epigenetic derepression of the Sry gene at a specific time of embryo development. Sry derepression is mediated by an iron-dependent histone demethylase KDM3A. Okashita et al demonstrated that iron deficiency in embryonic gonads decreases the ability of KDM3A to remove repressive histone marks from the Sry locus, reducing expression of Sry in those cells, and causing some XY embryos to develop ovaries.
Iron deficiency can cause male-to-female embryo sex reversal. Testis formation is initiated by epigenetic derepression of the Sry gene at a specific time of embryo development. Sry derepression is mediated by an iron-dependent histone demethylase KDM3A. Okashita et al demonstrated that iron deficiency in embryonic gonads decreases the ability of KDM3A to remove repressive histone marks from the Sry locus, reducing expression of Sry in those cells, and causing some XY embryos to develop ovaries.
The proposed mechanism of male-to-female sex reversal in mice appears to require severe gonadal iron deficiency for a penetrant phenotype. Nevertheless, even without complete sex reversal, altered KDM3A function in response to iron deficiency may subtly affect XY offspring. It remains to be determined whether these animals can reproduce normally, or whether other demethylases are impacted, and how that affects offspring development. Additionally, as prior research has shown that hypoxia itself can regulate KDM3A function,9 it is possible that sex reversal in iron deficiency anemia is mediated by tissue hypoxia, or its combination with iron deficiency.
Whether the findings are applicable to human pregnancies remains to be determined. Nevertheless, the demonstration that mammalian sex determination can be modulated by an environmental factor is a fundamental contribution to our understanding of development and reproduction.
The findings also raise an intriguing possibility that iron deficiency could contribute to the etiology of some human 46,XY female cases. Although the condition is known to be caused by androgen insensitivity syndrome (X-linked androgen receptor mutations) or gonadal dysgenesis (often linked to mutations in SRY), a considerable number of 46,XY female cases still have no identifiable genetic cause.10
In summary, the study highlights that iron-containing enzymes play a critical role in epigenetic processes, including embryonic sex determination, and raises the possibility that additional manifestations of iron deficiency may also be caused by impaired epigenetic regulation.
Conflict-of-interest disclosure: E.N. is a shareholder of and scientific adviser for Intrinsic LifeSciences; and consultant for Ionis Pharmaceuticals, Disc Medicine, Chugai, and Vifor. V.S. declares no competing financial interests.