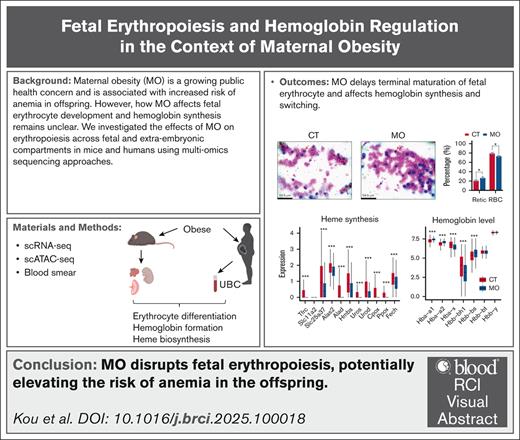
Key Points
MO accelerates fetal erythroid differentiation but impairs terminal maturation.
MO affects fetal hemoglobin synthesis and switching process.
Visual Abstract
Maternal obesity (MO) has been associated with an increased risk of anemia in infants and children; however, the underlying mechanisms remain poorly understood. To address this, we conducted single-cell RNA sequencing (scRNA-seq) on embryonic tissues involved in erythropoiesis, including the embryo proper, fetal liver, placenta, and yolk sac, across multiple developmental stages (embryonic day 9.5 [E9.5], E11.5, E13.5, and E17.5) in mouse models of MO. To further assess epigenetic regulation, we performed single-cell Assay for Transposase-Accessible Chromatin sequencing (scATAC-seq) on E13.5 embryos. We next validated the single-cell findings using publicly available scRNA-seq data from human umbilical cord blood (UCB) collected from neonates born to lean and obese mothers. Additionally, erythrocyte composition at different developmental stages (E13.5, E17.5, and postnatal day 0) was evaluated through blood smear analysis. Our data revealed that MO induced fetal hypoxia, which enhanced embryonic erythropoiesis via activation of the hypoxia-inducible factor 1α–erythropoietin signaling axis. Although MO promoted erythroid output and accelerated early differentiation, it concurrently impaired terminal erythrocyte maturation. Furthermore, MO delayed β-globin switching, with minimal effects on α-globin switching in fetal liver and human UCB erythrocytes. Notably, MO markedly reduced hemoglobin gene expression in both the mouse placenta and human UCB erythrocytes Collectively, these data demonstrated that MO disrupted fetal erythropoiesis and impaired erythrocyte maturation, potentially increasing the risk of anemia and related developmental consequences in offspring.
Introduction
Obesity is a global pandemic. According to the Centers for Disease Control and Prevention, >40% of American adults are obese,1 which is correlated with the incidences of type 2 diabetes, high blood pressure, metabolic syndrome, and other conditions.2 These comorbidities not only affect the mother but also affect fetal development, which can have serious health consequences for the offspring, including increased risks of stillbirth, congenital malformations, and anemia.3-5 Anemia is one of the most frequent disorders in infants and young children, which mainly involves a decrease in the number of circulating red blood cells (RBCs) or lower than normal hemoglobin levels.6 A prospective cohort study involving 17 193 mother-infant pairs demonstrated that a high maternal body mass index during pregnancy was associated with an increased risk of anemia in infants.4 However, due to ethical and technical limitations, particularly during the early developmental stages, studies on human erythropoiesis remain scarce. As a result, the precise mechanisms by which maternal obesity (MO) induces neonatal anemia remain unclear.
In both humans and mice, erythrocytes are the first blood cell type to emerge during embryogenesis, following broadly conserved developmental trajectories from extraembryonic (eg, yolk sac) to intraembryonic hematopoietic sites (eg, fetal liver and bone marrow). In mice, embryonic erythropoiesis occurs in 3 overlapping waves: (1) primitive erythropoiesis originating from yolk sac blood islands around embryonic day 7.25 (E7.25), producing primitive erythrocytes (EryPs); (2) prodefinitive erythropoiesis from yolk sac–derived erythromyeloid progenitors emerging at ∼E8.25 and colonizing the fetal liver by E9.5 to E10.5, generating early definitive erythrocytes (EryDs); and (3) definitive erythropoiesis from hematopoietic stem cells arising in the aorta-gonad-mesonephros region at ∼E10.5 and contributing to liver hematopoiesis by E12.5 to E13.5.7-9 In humans, EryPs first emerge in the yolk sac blood islands around gestational weeks 2 to 3. A comparable developmental transition then occurs, with erythropoiesis shifting to the fetal liver between weeks 4 to 6 and subsequently to the bone marrow by weeks 10 to 12.7,10,11
A previous study has clarified the dynamic contributions of each wave to erythroid populations at different developmental stages. At E9.5, circulating erythrocytes are predominantly EryPs. By E13.5, there is ∼60:40 ratio of EryPs to EryDs in circulation, shifting to ∼50:50 by E14.5. By E17.5, circulating erythrocytes are almost entirely EryDs.12 At E13.5, all active erythropoiesis takes place in the fetal liver, primarily generating erythromyeloid progenitor–derived EryDs, whereas circulating EryPs continue their maturation and enucleation. By E17.5, the erythropoietic program in the fetal liver has predominantly transitioned to hematopoietic stem cell–derived EryDs.9 Other extraembryonic hematopoietic tissues do not serve as active sites of erythropoiesis at this stage and contain only circulating erythroid cells. EryPs and EryDs share broadly similar maturation trajectories, involving progressive nuclear condensation, hemoglobinization, and eventual enucleation to form mature RBCs.13
Erythrocyte formation is tightly regulated by hypoxia-inducible factors (HIFs),14 which are composed of α and β subunits.15 HIF-1α and HIF-2α are considered the major isoforms involved in erythropoiesis regulation.16 Under normoxia, HIF-α subunits are hydroxylated and degraded; however, under hypoxia, they are stabilized, dimerize with HIF-β subunits, translocate to the nucleus, and activate erythropoiesis-related genes.17 Hypoxia-induced HIF activation promotes erythropoietin (EPO) production (kidney in adults and fetal liver in embryos),18 which binds EPO receptor to drive erythropoiesis.19
To date, it remains unclear how MO affects embryonic erythropoiesis and whether it contributes to neonatal anemia. In this study, using a well-established MO model of mice induced by high-fat diet feeding, we systematically profiled erythroid development in fetal mice by using single-cell RNA sequencing (scRNA-seq) and single-cell ATAC sequencing (scATAC-seq). In addition, related changes were further verified using scRNA-seq data from umbilical cord blood (UCB) of obese and lean mothers. We found that MO impaired erythrocyte development and affected hemoglobin synthesis in humans and mouse fetus, providing an explanation for anemia in neonates and children born to obese mothers.
Methods
MO induction in mice
C57BL/6 female mice were randomly separated into 2 groups that were fed either a control diet (10% energy from fat) or a high-fat diet (45% energy from fat). When the difference in body weight between the 2 groups exceeded 20%, female mice were mated with males fed a regular diet, and embryonic samples were obtained as previously described.20,21 The changes in maternal body weight, litter size, and fetal body weight are presented in supplemental Figure 4A-B.
scRNA-seq and scATAC-seq analyses
For scRNA-seq, embryonic livers were collected at E13.5 and E17.5, and placentas with attached yolk sacs were collected at E13.5. Samples were obtained from 3 randomly selected pregnant mice per group, with 3 embryos randomly selected from each pregnancy. Subsequently, single-cell suspensions were made as previously described.22 Libraries were generated using the Chromium GEM-X Single Cell 3′ Kit v4 (10x Genomics; PN-1000691), following the manufacturer’s protocol. Sequencing was performed on the Illumina NextSeq 2000 system (Illumina) using paired-end sequencing for the 3′ end transcriptome library. For scATAC-seq, embryonic proper tissues were collected at E13.5 from 3 pregnancies per group, with 3 embryos randomly selected from each pregnancy, followed by single-cell dissociation and nuclei isolation. Library preparation and sequencing were performed as previously described.23 In addition, E9.5, E11.5, and E13.5 embryos were used for scRNA-seq as described previously.20,21 The scRNA-seq data for adult mouse erythrocytes were derived from a previous study in which mice were fed with chow diet.24 Detailed analysis procedures are provided in the supplemental Materials.
Human UCB data
In human participants, maternal body mass index ≤24 and >30 kg/m2 were classified as lean and obese, respectively, paralleling the control (CT) and MO groups in mice. The scRNA-seq data of human UCB, derived from a previous study, included samples from 4 lean and 3 obese mothers.25
Erythrocyte concentration and morphology analysis
Peripheral blood from embryos was obtained by severing the head and umbilical cord. Maternal blood was obtained via orbital exsanguination under deep anesthesia. Erythrocyte concentration was assessed using a hemocytometer (Hausser Scientific). Thin smears of peripheral blood and touch preparations of fetal liver were stained with either New Methylene Blue N (Ricca Chemical) or Giemsa stain (Spectrum Chemical).26 Erythrocyte morphology was then analyzed using the Leica Application Suite v4.5.0 under a DM2000 LED microscope (Leica Microsystems).
RT-qPCR
Total RNA was extracted using TRIzol reagent (Invitrogen). Complementary DNA was synthesized using an iScript cDNA Synthesis Kit (Bio-Rad), and quantitative reverse transcription polymerase chain reaction (RT-qPCR) was performed with SsoAdvanced Universal SYBR Green Supermix (Bio-Rad) on a Bio-Rad CFX Real-Time PCR System. Gene expression levels were calculated using the 2–ΔΔCt method. Primer sequence information is displayed in supplemental Table 1.
Western blot
Western blots were conducted as previously described.21 Rabbit anti-mouse HIF-1α (1:800; Invitrogen) and mouse anti-mouse β-tubulin (1:1000; Invitrogen) were used for primary antibodies. Signal detection was performed using the Odyssey Infrared Imaging System (LI-COR Biosciences).
Flow cytometry
Flow cytometry was performed with modifications based on a previous study.12 Briefly, embryonic erythrocytes were collected and adjusted to 1 × 106 cells per mL. TER-119 (phycoerythrin) antibody (1:100; Invitrogen) was added to the erythrocyte suspension and analyzed using a Sony SH800 flow cytometry (Sony Biotechnology).
Statistical analysis
For scRNA-seq and scATAC-seq data, standard statistical approaches provided by the respective analysis packages were used without custom modifications. For measurements of erythrocyte concentration and the proportions of erythrocytes at different developmental stages in peripheral blood, as well as RT-qPCR and western blot analyses, unpaired 2-tailed Student t test was used. For all mouse experiments, 3 to 6 pregnancies per group were used depending on the assay, with each pregnancy as a biological replicate. A P value <.05 was considered statistically significant.
All animal experiments were conducted in accordance with the ethical standards approved by the Institutional Animal Care and Use Committee of Washington State University (protocol number 6704).
Result
MO induces embryonic hypoxia and erythropoiesis in mice
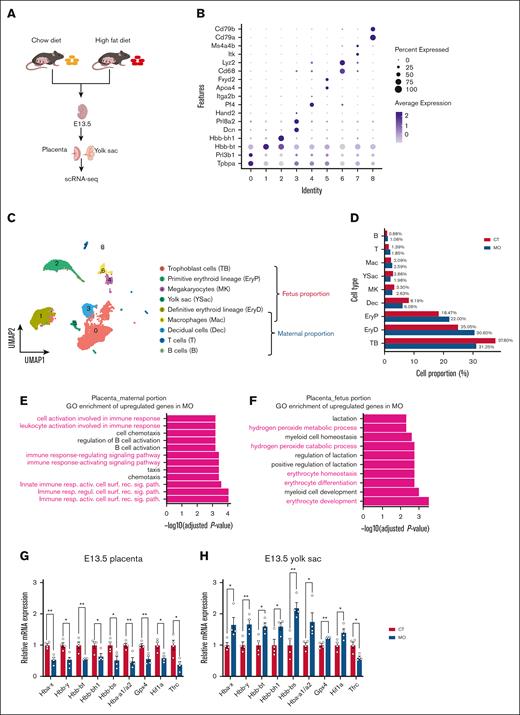
To investigate whether MO impairs placental function and leads to fetal hypoxia, we performed scRNA-seq on placentas and yolk sacs at E13.5 (Figure 1A). Based on specific cell markers (Figure 1B), we classified the placenta and yolk sac into maternal- and fetal-derived cell populations (Figure 1C). MO increased the proportions of EryPs and EryDs in the placenta and yolk sac (Figure 1D). Consistently, Gene Ontology (GO) enrichment of upregulated genes in MO indicated enhanced erythropoiesis-related processes in the fetal side (Figure 1E; false discovery rate [FDR, Benjamini–Hochberg–adjusted P value] < 0.05) but not in the maternal side (Figure 1F). Although erythrocyte proportion was increased in the placenta and yolk sac, hemoglobin gene expression showed tissue-specific differences. Based on RT-qPCR analysis, MO decreased hemoglobin gene expression in the placenta (Figure 1G; P < .05), but increased them in the yolk sac (Figure 1H; P < .05).
MO promotes erythropoiesis in E13.5 placenta and yolk sac. (A) Experimental workflow for scRNA-seq analysis of the E13.5 placenta and yolk sac. (B) Dot plot showing specific cell marker genes for each cluster. (C) UMAP of cell clusters, annotated into fetal and maternal compartments. (D) Bar plot displaying the proportions of different cell types in CT and MO groups. (E-F) GO enrichment analysis of genes upregulated in the MO group within the maternal (E) and fetal (F) compartments. (G-H) Quantitative RT-qPCR analysis of hemoglobin-related and erythropoiesis-associated gene expression in the placenta (G) and yolk sac (H) at E13.5. The scRNA-seq data were generated from pooled embryos (n = 3 pregnancies per group; 3 embryos per pregnancy). RT-qPCR was performed using embryos from 4 pregnancies per group. Data are presented as mean ± standard error of mean (SEM); each dot represents an individual embryo. Statistical significance was determined by unpaired 2-tailed t test. ∗P < .05; ∗∗P < .01. UMAP, uniform manifold approximation and projection.
MO promotes erythropoiesis in E13.5 placenta and yolk sac. (A) Experimental workflow for scRNA-seq analysis of the E13.5 placenta and yolk sac. (B) Dot plot showing specific cell marker genes for each cluster. (C) UMAP of cell clusters, annotated into fetal and maternal compartments. (D) Bar plot displaying the proportions of different cell types in CT and MO groups. (E-F) GO enrichment analysis of genes upregulated in the MO group within the maternal (E) and fetal (F) compartments. (G-H) Quantitative RT-qPCR analysis of hemoglobin-related and erythropoiesis-associated gene expression in the placenta (G) and yolk sac (H) at E13.5. The scRNA-seq data were generated from pooled embryos (n = 3 pregnancies per group; 3 embryos per pregnancy). RT-qPCR was performed using embryos from 4 pregnancies per group. Data are presented as mean ± standard error of mean (SEM); each dot represents an individual embryo. Statistical significance was determined by unpaired 2-tailed t test. ∗P < .05; ∗∗P < .01. UMAP, uniform manifold approximation and projection.
To further explore, we performed scRNA-seq on the embryo proper at E9.5, E11.5, and E13.5 in mice (supplemental Figure 1A-C). MO promoted hypoxia-related process as shown in gene set enrichment analysis (GSEA; supplemental Figure 1D-F) and increased Hif1a and Arnt (Hif1b) expression (supplemental Figure 1G-J; P < .05). Accordingly, scATAC-seq data of the embryo proper at E13.5 also showed that MO increased chromatin accessibility of Hif1a (supplemental Figure 1K-L). In addition, similar to the placenta and yolk sac, MO increased the proportions of EryDs and EryPs in the embryo proper (supplemental Figure 2A-F). Consistent with the yolk sac results, MO also elevated hemoglobin gene expression in erythroid cells within the embryo proper during E9.5 to E13.5 (supplemental Figure 2G; P < .05). These data demonstrated that MO induced embryo hypoxia and promoted erythropoiesis.
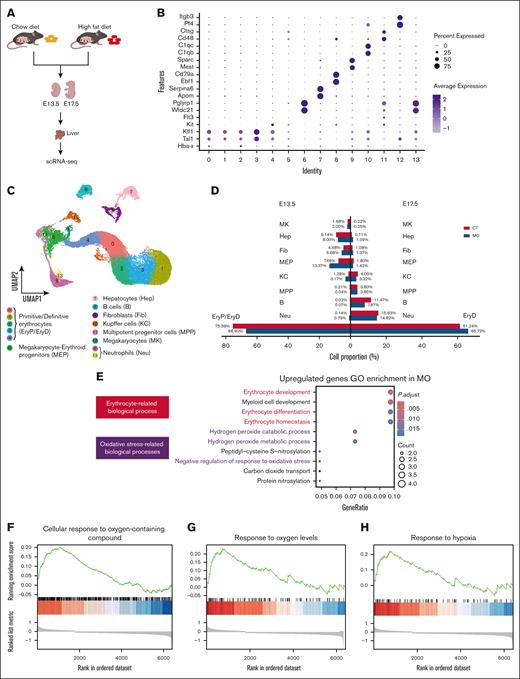
To investigate whether hypoxia affects embryonic liver erythropoiesis, we performed scRNA-seq on embryonic liver at E13.5 and E17.5 (Figure 2A-C). The E13.5 liver contains 2 erythroid populations, EryPs and EryDs, whereas the E17.5 liver harbors only EryDs (Figure 2D; supplemental Figure 7). Similar to the embryo proper, MO promoted the erythropoiesis process (Figure 2E; FDR < 0.05) and enhanced the hypoxia-related process (Figure 2F-H). To further confirm, we analyzed erythrocyte counts of fetal peripheral blood, showing elevated erythrocyte concentrations at E13.5, E17, and P0 (supplemental Figure 4C-E; P < .05). Overall, these data suggested that MO induced embryonic hypoxia and promoted erythropoiesis.
MO induces hypoxia stress and promotes erythropoiesis in E13.5 and E17.5 embryonic liver. (A) Experimental workflow for scRNA-seq of the E13.5 and E17.5 fetal livers from CT and MO groups. (B) Dot plot showing specific cell marker genes for each cluster. (C) UMAP projection displayed major hepatic and hematopoietic cell types. (D) Bar plot comparing cell type proportions between CT and MO groups. (E) GO enrichment analysis of upregulated genes in MO group, highlighting erythroid development and oxidative stress-related processes. (F-H) GSEA shows positive enrichment of oxygen-related response pathways in MO fetal liver cells. The scRNA-seq was performed using pooled embryos (n = 3 pregnancies per group; 3 embryos per pregnancy) for both E13.5 and E17.5 time points. UMAP, uniform manifold approximation and projection.
MO induces hypoxia stress and promotes erythropoiesis in E13.5 and E17.5 embryonic liver. (A) Experimental workflow for scRNA-seq of the E13.5 and E17.5 fetal livers from CT and MO groups. (B) Dot plot showing specific cell marker genes for each cluster. (C) UMAP projection displayed major hepatic and hematopoietic cell types. (D) Bar plot comparing cell type proportions between CT and MO groups. (E) GO enrichment analysis of upregulated genes in MO group, highlighting erythroid development and oxidative stress-related processes. (F-H) GSEA shows positive enrichment of oxygen-related response pathways in MO fetal liver cells. The scRNA-seq was performed using pooled embryos (n = 3 pregnancies per group; 3 embryos per pregnancy) for both E13.5 and E17.5 time points. UMAP, uniform manifold approximation and projection.
MO alters erythroid output and oxygen-sensing signaling in human UCB. (A) Overview of maternal characteristics. (B) Dot plot shows specific marker genes for each cluster. (C) UMAP plot colored by individual donor identity (top) and cell type annotations (bottom). (D) Violin plots of characteristic gene expression in clusters 4 and 7 (left) and bar plots of their proportions across conditions (right). (E) GO enrichment analysis of upregulated genes in the obese group. (F-G) GSEA plots showing positive enrichment of erythroid development and homeostasis pathways in the obese group. (H-J) GSEA plots showing negative enrichment of hypoxia and oxygen response pathways in the obese group. The scRNA-seq was performed on UCB samples from 3 obese and 4 lean human pregnancies. UMAP, uniform manifold approximation and projection.
MO alters erythroid output and oxygen-sensing signaling in human UCB. (A) Overview of maternal characteristics. (B) Dot plot shows specific marker genes for each cluster. (C) UMAP plot colored by individual donor identity (top) and cell type annotations (bottom). (D) Violin plots of characteristic gene expression in clusters 4 and 7 (left) and bar plots of their proportions across conditions (right). (E) GO enrichment analysis of upregulated genes in the obese group. (F-G) GSEA plots showing positive enrichment of erythroid development and homeostasis pathways in the obese group. (H-J) GSEA plots showing negative enrichment of hypoxia and oxygen response pathways in the obese group. The scRNA-seq was performed on UCB samples from 3 obese and 4 lean human pregnancies. UMAP, uniform manifold approximation and projection.
MO promotes erythropoiesis but disrupts its function in human UCB
In the scRNA-seq analysis of human UCB (Figure 3A-C), we also found that MO significantly increased the proportion of reticulocytes and nucleated RBCs (nRBCs) in humans (Figure 3D; P < .001) and enhanced erythropoiesis process (Figure 3E-G; FDR < 0.05). Consistently, clinical measurements of UCB revealed elevated concentrations of EPO27,28 and total hemoglobin29 in the obese group. We also observed that oxygen-sensing pathways were downregulated in UCB from the obese group, as indicated by negative enrichment in GSEA (Figure 3H-J; FDR < 0.05). This dysregulation may impair proper hypoxia responses. In agreement with this, clinical blood gas measurements revealed that obesity significantly reduced pO2, oxygen saturation, and total oxygen content in UCB.30 In short, these data suggested that although erythropoiesis was elevated, the functional capacity of erythroid cells was likely impaired in the UCB of obese pregnancies. This may be attributed to the increased presence of immature circulating nRBCs.
MO promotes embryonic erythropoiesis via the HIF-1α-EPO pathway
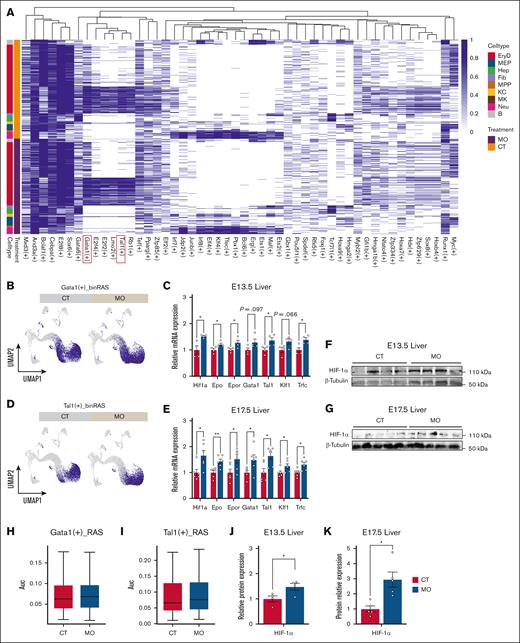
To explore whether MO promotes embryonic erythropoiesis via the HIF-1α-EPO pathway, we analyzed scATAC-seq data of erythrocytes derived from the E13.5 populations. We observed increased chromatin accessibility in key genes of the HIF-1α-EPO axis, including Hif1a, Epor, and Gata1 in the MO group (supplemental Figure 6A). In addition to these genes identified by scATAC-seq, we also observed elevated mRNA expression of Epo and Trfc in the fetal liver from MO group (Figure 4C,E; P < .05). Consistent with the gene expression level of Hif1a, MO also increased HIF-1α protein content in the embryonic liver (Figure 4F-G,J-K; P < .05). Moreover, we applied the Single-Cell rEgulatory Network Inference and Clustering (SCENIC) framework to reconstruct transcription factor regulatory networks using scRNA-seq data from the fetal liver at E13.5 and E17.5 (Figure 4A). The activity of key erythropoiesis-related regulators, including Gata1(+) and Tal1(+), was upregulated in the MO group (Figure 4B,D,H-I). These data suggested that MO promotes embryonic erythropoiesis via the HIF-1α-EPO pathway.
MO activates the HIF-1α-EPO signaling pathway and enhances erythroid regulatory networks. (A) Binarized regulon activity heat map of the top 50 transcription factors in E13.5 and E17.5 fetal liver, colored by cell type and treatment group. (B, D, H-I) UMAP visualization of Gata1 (B) and Tal1 (D) regulon activities in erythroid cells. Quantification of Gata1 (H) and Tal1 (I) regulon activities (AUC scores) in CT and MO groups. (C and E) RT-qPCR analysis shows upregulation of the mRNA levels of Hif1a, Epo, Epor, Gata1, Tal1, Klf1 and Trfc in E13.5 (C) and E17.5 (E) fetal livers. (F, G, J, K) Western blot bands showing HIF-1α protein levels in E13.5 (F) and E17.5 (G) fetal livers. Quantification of HIF-1α expression relative to β-tubulin in E13.5 (J) and E17.5 (K) fetal livers. The scRNA-seq analyses were based on pooled embryos from 3 pregnancies per group (3 embryos per pregnancy). RT-qPCR and western blot analyses were conducted using embryos from 4 to 6 pregnancies per group. Data are presented as mean ± SEM; each dot represents an individual embryo. Statistical significance was determined by unpaired 2-tailed t test. ∗P < .05; ∗∗P < .01. UMAP, uniform manifold approximation and projection.
MO activates the HIF-1α-EPO signaling pathway and enhances erythroid regulatory networks. (A) Binarized regulon activity heat map of the top 50 transcription factors in E13.5 and E17.5 fetal liver, colored by cell type and treatment group. (B, D, H-I) UMAP visualization of Gata1 (B) and Tal1 (D) regulon activities in erythroid cells. Quantification of Gata1 (H) and Tal1 (I) regulon activities (AUC scores) in CT and MO groups. (C and E) RT-qPCR analysis shows upregulation of the mRNA levels of Hif1a, Epo, Epor, Gata1, Tal1, Klf1 and Trfc in E13.5 (C) and E17.5 (E) fetal livers. (F, G, J, K) Western blot bands showing HIF-1α protein levels in E13.5 (F) and E17.5 (G) fetal livers. Quantification of HIF-1α expression relative to β-tubulin in E13.5 (J) and E17.5 (K) fetal livers. The scRNA-seq analyses were based on pooled embryos from 3 pregnancies per group (3 embryos per pregnancy). RT-qPCR and western blot analyses were conducted using embryos from 4 to 6 pregnancies per group. Data are presented as mean ± SEM; each dot represents an individual embryo. Statistical significance was determined by unpaired 2-tailed t test. ∗P < .05; ∗∗P < .01. UMAP, uniform manifold approximation and projection.
MO accelerates fetal erythrocyte differentiation but impairs terminal maturation
To explore whether MO affects fetal erythrocyte differentiation, we performed development trajectory analysis using scRNA-seq data. First, erythroid subclusters were extracted from the mouse placenta and yolk sac at E13.5 (EryPs), embryonic liver at E17.5 (EryDs) and human UCB (nRBCs) as shown in Figure 5A-C. Then, we reconstructed differentiation trajectories by using pseudotime analysis31,32 (Figure 5D-F). By comparing the expression patterns of stage-specific genes22,33 (supplemental Figure 3), we assigned erythroid cells to distinct developmental stages along the trajectories (Figure 5G-I). As shown in Figure 5J-O, MO increased the proportion of late-stage erythroid populations and decreased the proportion of early-stage erythroid populations, suggesting that MO accelerated erythroid differentiation in both mice and human UCB. For EryPs and nRBCs, the predominance of cells in the gap 1 (G1) phase (Figure 5P,T), along with the loss of Pcna/PCNA and Mki67/MKI67 expression (Figure 5Q,U), shows that most cells had exited the cell cycle and reached a terminally differentiated state for both MO/obese and CT/lean groups. In addition, an increased proportion of EryDs in the S and G2/M phases but a reduced proportion in the G1 phase was observed in the MO group (Figure 5R), along with elevated expression of Pcna and Mki67 (Figure 5S), suggesting that MO increased EryDs proliferation in fetal liver.
MO accelerates erythroid differentiation in mice and human UCB. (A-C) UMAP plots of erythroid cells from the placenta and yolk sac (A), fetal liver (B), and human UCB (C), colored by identified erythroid subclusters. (D-F) Pseudotime trajectory analysis of erythroid cells from the mice and human UCB. (G-I) Violin plots shows the expression patterns of hemoglobin genes, erythroid transcription factors, nuclear-related genes, and total gene counts (nFeature_RNA) across erythroid subclusters. (J-O) Pie charts showing shifts in erythroid subpopulation distributions between the CT and MO (or lean and obese) groups. (P,R,T) Bar plots of G1/S/G2M phase proportions. (Q,S,U) Ridge plots of Pcna/PCNA and Mki67/MKI67 expression levels. The scRNA-seq analyses were based on 3 pregnancies per group for mouse tissues (3 embryos per pregnancy) and 4 lean vs 3 obese samples for human UCB. BFU-E, burst-forming unit–erythroid; CFU-E, colony-forming unit–erythroid; ProE, proerythroblast; BasoE, basophilic erythroblast; PolyE, polychromatic erythroblast; OrthoE, orthochromatic erythroblast; Retic, reticulocyte.
MO accelerates erythroid differentiation in mice and human UCB. (A-C) UMAP plots of erythroid cells from the placenta and yolk sac (A), fetal liver (B), and human UCB (C), colored by identified erythroid subclusters. (D-F) Pseudotime trajectory analysis of erythroid cells from the mice and human UCB. (G-I) Violin plots shows the expression patterns of hemoglobin genes, erythroid transcription factors, nuclear-related genes, and total gene counts (nFeature_RNA) across erythroid subclusters. (J-O) Pie charts showing shifts in erythroid subpopulation distributions between the CT and MO (or lean and obese) groups. (P,R,T) Bar plots of G1/S/G2M phase proportions. (Q,S,U) Ridge plots of Pcna/PCNA and Mki67/MKI67 expression levels. The scRNA-seq analyses were based on 3 pregnancies per group for mouse tissues (3 embryos per pregnancy) and 4 lean vs 3 obese samples for human UCB. BFU-E, burst-forming unit–erythroid; CFU-E, colony-forming unit–erythroid; ProE, proerythroblast; BasoE, basophilic erythroblast; PolyE, polychromatic erythroblast; OrthoE, orthochromatic erythroblast; Retic, reticulocyte.
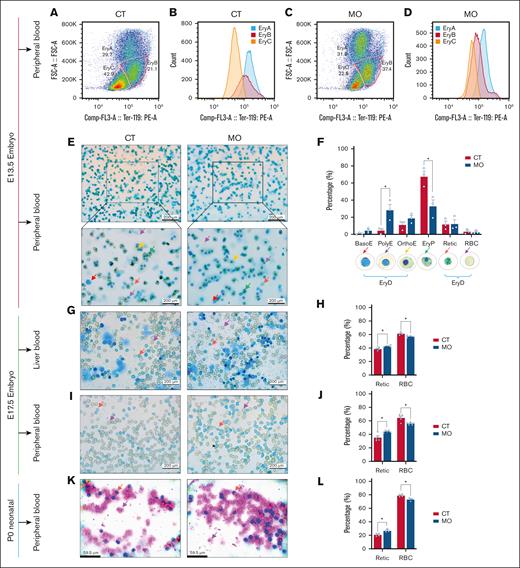
To further verify, we performed flow cytometry analysis of E13.5 fetal peripheral blood based on Ter119 expression and cell size (forward scatter area [FSC-A]). Three populations were identified: erythroblast A (EryA; large size and high Ter119), EryB (intermediate size and medium Ter119), and EryC (small size and low Ter119; Figure 6A-D). Based on a previous study reporting a EryPs:EryDs ratio of ∼69:31 at E13.5,12 we interpret EryB and EryC as corresponding primarily to the EryPs population, whereas EryA represents EryDs. In line with this interpretation, we observed a lower proportion of EryPs (EryB + EryC) and a higher proportion of EryDs (EryA) in the MO group than the CT group (Figure 6A,C), suggesting that MO promotes the expansion of definitive erythroid cells. Blood smear staining corroborated these findings. At E13.5, we further distinguished EryPs based on previously described features, including (1) cell morphology, (2) a nuclear-to-cytoplasmic diameter ratio of ∼0.1, and (3) the relative abundance of EryPs vs EryDs (∼69:31), as reported by previous studies.12,34 Our blood smear analysis, primarily from umbilical cord outflow, revealed that most erythroid cells at this stage were nucleated (Figure 6E). These nucleated cells exhibited morphological features and relative abundance consistent with EryPs identity. Furthermore, MO fetuses displayed a higher proportion of early-stage EryDs and a reduced proportion of EryPs (Figure 6F), suggesting that MO delayed the terminal maturation of EryD. This trend persisted into later developmental stages. At E17.5 (Figure 6G-J) and P0 (Figure 6K-L), MO fetuses continued to have a higher proportion of reticulocytes and a lower proportion of RBCs (P < .05).
MO delays terminal maturation in mouse embryonic erythrocyte. (A-D) Flow cytometry analysis of fetal peripheral blood at E13.5, identifying EryA, EryB, and EryC populations based on FSC-A and Ter119 expression. (E) Representative peripheral blood smears stained with new methylene blue at E13.5. The top panels were imaged at 100× magnification and the bottom panels at 400× magnification. (F) Proportional analysis of erythroid cells in peripheral blood at E13.5. (G-H) Representative images of new methylene blue–stained liver blood cells at E17.5 (G, 400× magnification), with corresponding quantification (H). (I-J) Representative images of new methylene blue staining in peripheral blood cells at E17.5 (I, 400× magnification), and quantification of the staining (J). (K-L) Representative images of Giemsa-stained neonatal peripheral blood cells at P0 (K, 400× magnification), with corresponding quantification (L). Data are presented as mean ± SEM; each dot represents an individual embryo. For all experiments, embryos from 3 to 6 pregnancies per group were used. For flow cytometry, one embryo was selected from each pregnancy, and fetal blood from these embryos was pooled within each group (CT or MO) to generate a single sample for analysis. Statistical significance was determined by unpaired 2-tailed t test. ∗P < .05. Colored arrows in the representative images indicate typical erythrocytes at different developmental stages. BasoE, basophilic erythroblast; PolyE, polychromatic erythroblast; OrthoE, orthochromatic erythroblast; Retic, reticulocyte.
MO delays terminal maturation in mouse embryonic erythrocyte. (A-D) Flow cytometry analysis of fetal peripheral blood at E13.5, identifying EryA, EryB, and EryC populations based on FSC-A and Ter119 expression. (E) Representative peripheral blood smears stained with new methylene blue at E13.5. The top panels were imaged at 100× magnification and the bottom panels at 400× magnification. (F) Proportional analysis of erythroid cells in peripheral blood at E13.5. (G-H) Representative images of new methylene blue–stained liver blood cells at E17.5 (G, 400× magnification), with corresponding quantification (H). (I-J) Representative images of new methylene blue staining in peripheral blood cells at E17.5 (I, 400× magnification), and quantification of the staining (J). (K-L) Representative images of Giemsa-stained neonatal peripheral blood cells at P0 (K, 400× magnification), with corresponding quantification (L). Data are presented as mean ± SEM; each dot represents an individual embryo. For all experiments, embryos from 3 to 6 pregnancies per group were used. For flow cytometry, one embryo was selected from each pregnancy, and fetal blood from these embryos was pooled within each group (CT or MO) to generate a single sample for analysis. Statistical significance was determined by unpaired 2-tailed t test. ∗P < .05. Colored arrows in the representative images indicate typical erythrocytes at different developmental stages. BasoE, basophilic erythroblast; PolyE, polychromatic erythroblast; OrthoE, orthochromatic erythroblast; Retic, reticulocyte.
In humans, similar to the findings in mouse fetal peripheral blood, MO markedly increased the proportion of nRBCs in UCB,28 suggesting that MO impaired terminal erythroid maturation in human fetuses.
MO affects fetal hemoglobin synthesis and switching process
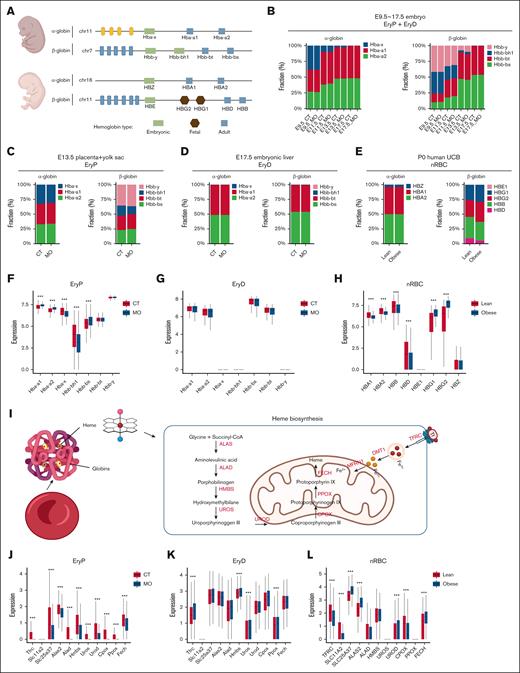
To assess whether MO affects hemoglobin synthesis and switching, we analyzed scRNA-seq data from EryPs, EryDs, and nRBCs in mice and humans. The sequential conversion of α- and β-globin gene families from embryonic to adult hemoglobin during development35 is shown in Figure 7A. EryDs exclusively expressed adult hemoglobin (Figure 7D; supplemental Figure 5E), whereas EryPs and nRBCs expressed a combination of embryonic, fetal (human only), and adult hemoglobin (Figure 7C,E). MO delayed β-globin switching, whereas α-globin transition remained largely unaffected in total erythrocytes (EryDs + EryPs) isolated from the embryo proper at E13.5 in mice (Figure 7B).
MO disrupts hemoglobin switching and heme biosynthesis in fetal erythroid cells. (A) Schematic overview of α- and β-globin gene clusters and their developmental expression patterns in mice and humans. (B) Hemoglobin switching dynamics from embryonic to adult forms in mouse embryos (E9.5-E17.5). (C-E) Bar plots show α- and β-globin composition in mouse placenta with yolk sac (EryPs), embryonic liver (EryDs), and human UCB erythroid cells (nRBCs). (F-H) Expression levels of globin genes in EryPs, EryDs, and nRBCs. (I) Schematic diagram illustrating iron transport and mitochondrial heme biosynthesis pathways. (J-L) Expression of key genes involved in iron transportation (TFRC/Tfrc, SLC11A2/Slc11a2, and SLC25A37/Slc25a37) and heme biosynthesis (ALAS2/Alas2, HMBS/Hmbs, UROS/Uros, UROD/Urod, CPOX/Cpox, PPOX/Ppox, and FECH/Fech) in EryPs, EryDs, and nRBCs. The scRNA-seq analyses were based on 3 pregnancies per group for mouse tissues (3 embryos per pregnancy) and 4 lean vs 3 obese samples for human UCB. Statistical significance was determined using the Wilcoxon rank-sum test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
MO disrupts hemoglobin switching and heme biosynthesis in fetal erythroid cells. (A) Schematic overview of α- and β-globin gene clusters and their developmental expression patterns in mice and humans. (B) Hemoglobin switching dynamics from embryonic to adult forms in mouse embryos (E9.5-E17.5). (C-E) Bar plots show α- and β-globin composition in mouse placenta with yolk sac (EryPs), embryonic liver (EryDs), and human UCB erythroid cells (nRBCs). (F-H) Expression levels of globin genes in EryPs, EryDs, and nRBCs. (I) Schematic diagram illustrating iron transport and mitochondrial heme biosynthesis pathways. (J-L) Expression of key genes involved in iron transportation (TFRC/Tfrc, SLC11A2/Slc11a2, and SLC25A37/Slc25a37) and heme biosynthesis (ALAS2/Alas2, HMBS/Hmbs, UROS/Uros, UROD/Urod, CPOX/Cpox, PPOX/Ppox, and FECH/Fech) in EryPs, EryDs, and nRBCs. The scRNA-seq analyses were based on 3 pregnancies per group for mouse tissues (3 embryos per pregnancy) and 4 lean vs 3 obese samples for human UCB. Statistical significance was determined using the Wilcoxon rank-sum test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
To assess whether MO exerts similar effects on erythrocytes of different origins, we analyzed the composition of α- and β-globin in EryDs, EryPs, and nRBCs. In mice, MO promoted β-globin switching in EryPs from the placenta with yolk sac, as indicated by the increased proportion of adult β-globins but decreased embryonic β-globins (Figure 7C). In contrast, MO delayed β-globin switching in EryPs from the embryonic liver (supplemental Figure 8B). Interestingly, MO did not affect α- or β-globin composition in EryDs (Figure 7D; supplemental Figure 8B). In humans, MO increased the fraction of fetal β-globins but reduced adult β-globins in nRBCs (Figure 7E), indicating a delayed switching process. Notably, α-globin switching process was unaffected in both mouse and human erythrocytes. These data showed that MO delayed β-globin switching in both mice and human erythrocytes.
To clarify the effects of MO on hemoglobin content in erythrocytes of different origins, we analyzed hemoglobin gene expression levels in EryDs, EryPs, and nRBCs. In mice, MO increased adult globin levels but decreased embryonic globin levels in EryPs (Figure 7F; supplemental Figure 8E; P < .0001). MO also increased adult globin levels in EryDs from the E13.5 liver (supplemental Figure 8F; P < .0001) but had no significant effects on hemoglobin gene expression in EryDs from the E17.5 liver (Figure 7G). In humans, MO remarkably increased fetal globin expression but reduced adult globin levels in nRBCs (Figure 7H; P < .0001). Although a previous clinical study had reported that MO increased total hemoglobin levels in UCB,29 the lower levels of adult hemoglobin might partially contribute to impaired oxygen transport. These data suggested that MO affected hemoglobin synthesis in both mouse and human fetuses, likely contributing to hypoxia in fetuses.
To further investigate whether MO affects heme synthesis, we explored the expression of key enzymes and iron transport-related genes involved in heme biosynthesis (Figure 7I). In mice, MO substantially upregulated key genes involved in heme biosynthesis and iron uptake in EryDs (Figure 7K; supplemental Figure 8H; P < .0001), which was consistent with increased hemoglobin in EryDs (supplemental Figure 8F). MO markedly suppressed the expression of most of these genes in EryPs and nRBCs (Figure 7J,L; P < .0001), which was consistent with decreased hemoglobin in EryPs and nRBCs. These data suggested that MO decreased heme biosynthesis in erythrocytes of the placenta, yolk sac, and human UCB, while enhancing heme production in erythrocytes from the embryonic liver.
Discussion
MO is known to impair placental structure and function through multiple pathways, including increased placental inflammation,36 oxidative stress,37 and vascular dysfunction.38 These alterations can reduce uteroplacental perfusion and oxygen transport efficiency and elevate the expression of hypoxia-responsive genes in fetal-facing trophoblasts.39,40 Additionally, excessive lipid accumulation and metabolic dysregulation in the placenta can disrupt mitochondrial function and impair oxygen utilization.41,42 These pathological changes may disrupt the maternal-placental-fetal oxygen axis, which plays a critical role in ensuring adequate oxygen delivery to support fetal development.43 Together, these alterations likely compromise oxygen exchange efficiency, ultimately contributing to a hypoxic intrauterine environment.
Previous studies have shown that MO induces fetal hypoxia.27,30,44 In this study, we found that MO reduced the expression levels of hemoglobin genes in the placenta, which may compromise oxygen transport to the fetus and contribute to fetal hypoxia. This was further supported by our mouse scRNA-seq and scATAC-seq data, which revealed upregulation of hypoxia-related biological processes and increased expression of Hif1a and Hif1b due to MO. Interestingly, we also observed that MO increased the expression of hemoglobin-related genes in the yolk sac and fetal liver at E13.5. This apparent discrepancy may reflect the complex cellular composition of placental erythroid cells, which include both maternal and fetal populations. Because the yolk sac and fetal liver are exclusively fetal in origin, the upregulation of hemoglobin gene expression in these tissues suggests that the fetal contribution in the placenta might have increased, whereas maternal-side expression declined, resulting in an overall reduction. This decrease in placental hemoglobin gene expression might represent an adaptive response to MO-induced hypoxia. In human UCB, PO2, oxygen content, and oxygen saturation were reported to be lower in obese mothers than in lean mothers,30 suggesting that human fetuses also experience hypoxia. Collectively, these findings show that MO induces fetal hypoxia in both mice and humans.
Hypoxia induces erythropoiesis via the HIFs-EPO axis.45-47 In this study, Hif1a and Hif1b were primarily expressed in mouse embryos based on scRNA-seq data. MO increased chromatin accessibility at key genes in the HIF-1α-EPO axis, including Hif1a and Epor. Correspondingly, MO upregulated the expression of these genes, as well as Epo and Trfc in the mouse fetal liver. These findings support that MO promotes embryonic erythropoiesis through activation of the HIF-1α-EPO pathway. The increased proportion of erythrocytes in mouse embryos in scRNA-seq data, together with elevated erythrocyte concentrations in fetal peripheral blood, further confirms that MO enhanced erythropoiesis. Similarly, in human UCB samples, elevated EPO levels,27,28 and erythrocyte proportion were observed in obese mothers in scRNA-seq data.
Previous studies have shown that hypoxia promoted erythrocyte differentiation.46,48 Consistently, in our study, scRNA-seq data revealed accelerated erythroid progression under MO conditions. The scRNA-seq analysis showed a reduction in early-stage and an increase in late-stage erythroid populations under MO conditions. These findings indicate that MO promotes erythroid differentiation. Because scRNA-seq cannot assess mature RBCs, we performed blood smears stained with new methylene blue and Giemsa.26 We found that MO increased the proportion of reticulocytes while reducing that of mature RBCs in fetal blood. Similarly, MO elevated the proportion of nRBCs in human UCB samples.28 These findings suggest that, despite the accelerated differentiation process, MO impairs terminal erythroid maturation. A recent study has suggested that impaired terminal erythroid maturation may be influenced by metabolic alterations beyond hypoxia. In particular, intracellular cholesterol accumulation has been shown to disrupt erythroblast enucleation and late-stage differentiation by activating the SREBP signaling pathway, whereas pharmacologic inhibition of cholesterol biosynthesis using fatostatin restores normal erythroid maturation.49 Given that MO is associated with dysregulated lipid metabolism and elevated cholesterol levels in the fetus, it is plausible that MO-induced cholesterol accumulation in erythroid progenitors contributes to the observed defects in terminal maturation. Thus, although MO-driven hypoxia may accelerate early erythroid differentiation, excess intracellular cholesterol may interfere with the final steps of erythropoiesis, resulting in an increased proportion of immature erythroid cells, such as reticulocytes and nRBCs. This dual effect may underlie the paradoxical combination of enhanced differentiation with impaired maturation observed in both mouse and human data sets.
Hemoglobin is composed of globin and heme,50 which gradually transition from embryonic to adult forms during embryonic development.13,35,51 In our study, MO had minimal impact on α-globin switching but delayed β-globin switching in both mouse embryonic and human UCB erythrocytes. Delayed transition from embryonic to adult β-globin during fetal development may impair the oxygen-carrying efficiency of erythrocytes, resulting in suboptimal oxygen delivery to fetal tissues. Hypoxia has been shown to upregulate hemoglobin.52,53 Consistently, MO upregulated the expression of most hemoglobin genes in EryDs of mouse fetal liver in our study. However, MO also induced widespread downregulation of hemoglobin genes in mouse EryPs and human nRBCs.
Heme binds oxygen through its central Fe2+, enabling hemoglobin function.54 A previous study has indicated that MO can cause embryonic iron deficiency,29 which may lead to impaired heme synthesis. In our study, MO significantly downregulated genes involved in heme synthesis in human UCB (nRBCs) and mouse placental and yolk sac (EryPs) erythrocytes based on scRNA-seq but had a promoting effect on erythrocyte production in mouse embryonic liver (EryDs). In addition, MO also accelerated erythroid differentiation, which might have contributed to the downregulation of heme biosynthetic genes observed in placental and UCB erythroid cells. As erythrocytes undergo terminal differentiation, especially during mid-to-late EryPs or EryDs stages, transcriptional activity decreases due to nuclear condensation and enucleation, resulting in reduced expression of metabolic and biosynthetic genes, including those involved in heme production. In our study, MO promoted erythroid differentiation in the placenta with yolk sac and human UCB, as evidenced by increased proportions of late-stage erythroid cells, which may account for the diminished heme gene expression. In contrast, the embryonic liver retained a higher population of immature erythroid precursors under MO conditions, leading to elevated heme gene expression. Moreover, the upregulation of iron transporter gene Tfrc in the fetal liver further supports enhanced iron uptake and heme synthesis in this tissue. Together, these findings suggest that the differential effects of MO on heme biosynthesis may reflect tissue-specific differences in erythroid cell composition, maturation stage, and iron availability.
Conclusion
In summary, our study reveals that MO promotes erythropoiesis via hypoxia-induced activation of the HIF-1α-EPO signaling axis. Although MO accelerates erythroid differentiation, it impairs terminal maturation and disrupts heme and hemoglobin synthesis. It also delays β-globin switching, while α-globin switching remains unaffected. These alterations may collectively increase the risk of anemia in the offspring.
Acknowledgments
All scientific illustrations in the figures were created with BioRender.com. Kou, Z. (2025) https://BioRender.com/6metezc.
This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health grant R01HD067449.
Authorship
Contribution: Z.K. and M.D. designed the research; Z.K., S.I., X.L., C.S., Q.C., and J.M.d.A. performed the research; Z.K., M.N.H., and L.-W.C. analyzed data; M.-J.Z. and M.D. contributed vital analytical tools; and Z.K. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Min Du, Nutrigenomics and Growth Biology Laboratory, Department of Animal Sciences, Washington State University, Pullman, WA 99164; email: min.du@wsu.edu.
References
Author notes
The scRNA-seq and scATAC-seq data sets used in this study comprise both publicly available and newly generated data. Public data sets include embryonic scRNA-seq data at embryonic day 9.5 (E9.5), E11.5, and E13.5, and embryonic scATAC-seq data at E13.5 (Gene Expression Omnibus [GEO] accession numbers GSE173994 and GSE278631), as well as adult mouse peripheral blood scRNA-seq (GEO accession number GSE120505) and human fetal umbilical cord blood scRNA-seq (National Center for Biotechnology Information BioProject accession number PRJNA847067). Newly generated data sets include scRNA-seq of E13.5 and E17.5 embryonic liver and scRNA-seq of E13.5 placenta with yolk sac. These data sets have been deposited in the GEO database under accession number GSE303768.
The full-text version of this article contains a data supplement.