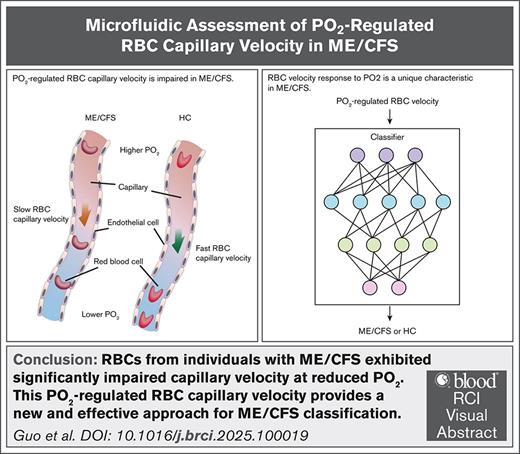
Key Points
PO2-regulated RBC capillary velocity is impaired in ME/CFS.
RBC velocity response to PO2 is a unique characteristic in ME/CFS.
Visual Abstract
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a complex disease of unknown etiology that affects multiple organ systems. Although there is no established treatment or diagnostic test for ME/CFS yet, studies have consistently demonstrated impaired cerebral blood flow (CBF) and blood flow regulation in ME/CFS. In this study, we measured red blood cell (RBC) velocity in microfluidic capillaries at varied oxygen tensions (PO2) and showed that, compared to RBCs from heathy controls, RBCs from patients with ME/CFS exhibit compromised capillary velocity in response to reduced PO2. To examine whether such PO2-regulated RBC capillary velocity could be used to assess or diagnose ME/CFS, we conducted receiver operator characteristic analysis and used machine learning to analyze various features of PO2-regulated RBC capillary velocity. We found that velocity slope–based classifiers were highly accurate, sensitive, and specific (ie, 77.8%, 76%, and 90%, respectively) in ME/CFS classification. Furthermore, we demonstrated this RBC-based microfluidic approach can be used to evaluate potential drugs (ie, salmeterol xinafoate and xanomeline) for improving RBC capillary velocity in ME/CFS. These findings highlight previously unrecognized roles of RBCs in the pathophysiology of ME/CFS and suggest a potential RBC-based test for ME/CFS diagnosis.
Introduction
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a complex, debilitating disease affecting >20 million people of all ages and races worldwide.1-3 ME/CFS is characterized by profound fatigue that is not alleviated by rest and persistent postexertional malaise. Additional symptoms include impaired memory, orthostatic intolerance, tender lymph nodes, unrefreshing sleep, headaches, and polyarthralgia, all of which are related to cognitive, immune, and autonomous dysfunctions.1,4-6 Approximately 70% of patients with ME/CFS cannot work, and at least 25% become bed- or house-bound for extended periods. Furthermore, up to 40% of COVID-19 survivors exhibited persistent, debilitating symptoms that resemble ME/CFS,7-10 raising concerns about a potential ME/CFS-like pandemic in this population (ie, long COVID).
The causes of ME/CFS are inconclusive, and no clinically accepted treatment and diagnostic biomarkers have been established.11 However, ME/CFS has been consistently associated with abnormal cerebral blood flow (CBF) and impaired blood flow regulation. Studies have shown that patients with ME/CFS exhibited reduced global12-14 and regional15 baseline CBF compared to healthy controls (HCs). Reduced baseline regional CBF in the anterior cingulate cortex was associated with the overall symptom severity score of ME/CFS,15 demonstrating a tight relation between impaired CBF and ME/CFS progression. Additionally, decreased CBF during orthostatic intolerance (a head-up tilt testing) has been observed in ME/CFS.16-20 The degree of CBF reduction during the tilt testing is also related to ME/CFS severity scores.21 Furthermore, functional magnetic resonance imaging and blood oxygenation level–dependent imaging have shown reduced brain responsiveness,22 delayed reaction time,23 and extended blood oxygenation level–dependent activation regions23 in ME/CFS, suggesting abnormal CBF regulation, that is, neurovascular coupling (NVC). Using an invasive cardiopulmonary exercise testing, peripheral neurovascular dysregulation, and impaired oxygen extraction were also observed in ME/CFS.24
The underlying mechanisms of impaired CBF and NVC in ME/CFS remain unclear. We have previously demonstrated that cerebral NVC in mouse brains is initiated in brain capillaries. In these capillaries, activity-induced decreases in local oxygen tension (PO2) trigger an increase of red blood cell (RBC) capillary velocity.25 This PO2-regulated RBC capillary velocity was further confirmed in ex vivo microfluidic capillaries, where reduced PO2 increases RBC deformability via a hemoglobin–band 3–based molecular switch on the RBC membrane, consequently increasing RBC capillary velocity.26 Thus, RBCs are active players in capillary blood flow, promptly increasing O2 delivery in response to activity-induced local changes in PO2. Because ME/CFS exhibits increased levels of inflammation and oxidative stress27-33 and RBCs are known to interact with cytokines34-37 and are susceptible to oxidative modifications,38-41 it is likely that RBC functions in ME/CFS are impaired, contributing to the widely observed CBF impairment. Herein, we measured ex vivo PO2-regulated capillary velocity of RBCs from patients with ME/CFS and HCs. We explored the use of PO2-regulated RBC capillary velocity for ME/CFS classification and tested salmeterol xinafoate and xanomeline as potential drugs to improve RBC velocity in ME/CFS. The results presented here unveil a previously unrealized role of RBC in ME/CFS and suggest a potential RBC-based approach for ME/CFS diagnosis and treatment.
Materials and methods
Measurement of PO2-regulated RBC capillary velocity
Microfluidic devices were fabricated using standard photolithography and polydimethylsiloxane soft-lithography processes. One-milliliter whole blood sample obtained from HCs or patients with ME/CFS (diagnosed based on the Canadian Consensus Criteria42) was centrifuged at 300g at room temperature for 1.5 minutes. The resulting RBC pallets were washed 3 times in phosphate-buffered saline (PBS; Thermo Fisher, Gibco; 20012-PBS; pH = 7.2). Nitrogen-purged PBS was prepared by introducing nitrogen to PBS for 3 hours on days receiving the blood samples. Then, 10 μL of packed RBCs were resuspended into 1 mL of nitrogen-purged PBS and introduced to the microfluidic device through a polyethylene tube (PE-20) under a consistent pressure (1.6 psi) provided by a nitrogen tank. An infusion pressure of 1.6 psi was selected to mimic the physiological arteriovenous pressure gradient, which is ∼90 mm Hg (equivalent to ∼1.7 psi).43 The microfluidic device had a 170-μm long constriction channel with dimensions of 5.05 ± 0.1 μm (width) × 5.94 ± 0.33 μm (height). The PO2 level inside the microfluidic channel was controlled by immersing the microfluidic device inside a sulfite sink (sodium sulfite; Millipore Sigma; S0505-250G) at concentrations of 0.0, 0.01, 0.1, and 1.0 M. PO2 in the microfluidic channel was colorimetrically quantified as described previously25 and in the supplemental Methods. RBC movements at each PO2 level were recorded by a high-speed camera (Phantom Miro 320), and RBC capillary velocity was calculated from image analysis using Image J and the Phantom Camera Control software. Each sample was analyzed using the same microfluidic chip across all 4 PO2 levels, provided the channel remained undamaged. These experiments were approved by the Stanford Institutional Review Board (IRB) and the University of California Davis IRB, and written consent was obtained before testing or analysis. This study was conducted in accordance with the Declaration of Helsinki and was approved by the University of California Davis IRB (protocol number 1332137-4).
Incubation RBCs with salmeterol xinafoate and xanomeline
Salmeterol xinafoate (Millipore Sigma; S5068-10MG) or xanomeline (Cayman Chemical; 10790) was dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich; 472301; 100 mL) to prepare a stock solution of 1 μM or 10 mM, respectively. The stock solution was then added to the RBC suspension in PBS (Thermo Fisher, Gibco; 20012-PBS; pH = 7.2) to achieve a concentration of 1 nM or 10 nM salmeterol xinafoate or 1 μM or 10 μM xanomeline. These RBC suspensions with salmeterol xinafoate or xanomeline were then incubated for 15 minutes at room temperature before testing.
Machine learning
Leave-one-subject-out (LOSO) evaluation was used to assess accuracy; details of the calculation were described in the supplemental Methods. To evaluate different feature sets, we conducted forward-feature selection, backward-feature selection, and domain knowledge–driven feature selection. The results are generated using the Scikit-learn 1.3.0 software package.
Statistical analysis
Data are reported as mean ± standard error of the mean. Statistical significance was set at a confidence level for all tests (P < .05), and Prism (GraphPad) was used for statistical analysis. Data shown in supplemental Figure 3 were compared using z tests. This manuscript will be deposited in PubMed Central, and data are available from the corresponding contacts upon request.
Results
PO2-regulated RBC capillary velocity is impaired in ME/CFS
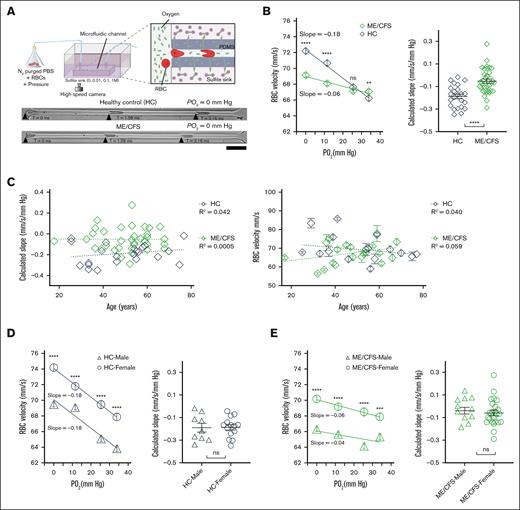
To examine PO2-regulated RBC capillary velocity in individuals with ME/CFS, we extracted RBCs from 35 patients with ME/CFS (aged 17-68 years; 25 females and 10 males) and 23 HC participants (aged 25-77 years; 14 females and 9 males; supplemental Tables 1 and 2) and measured RBC velocity at reduced PO2 levels. RBCs, suspended in N2-purged PBS, were injected under a consistent pressure (1.6 psi) into a microfluidic device immersed in an oxygen scavenger sink (sodium sulfite; Figure 1A). The microfluidic device had a constriction channel with dimensions of 5.05 ± 0.1 μm (width) × 5.94 ± 0.33 μm (height), mimicking the size of a typical capillary blood vessel.44 These variations in channel size did not lead to significant changes (∼3.9%) in volumetric flow rate. The oxygen scavenger sink contained 0.0, 0.01, 0.1, or 1.0 M of sodium sulfite, resulting in PO2 levels of 34 , 25, 12, or 0 mm Hg inside the microfluidic capillary, respectively.26 RBC movements under these PO2 levels were recorded by a high-speed camera and analyzed to obtain RBC velocity. The PO2 levels inside the microfluidic capillary are within a rage similar to that in the brain during normoxia (20-60 mm Hg) and hypoxia (2-18 mm Hg).45
RBCs from ME/CFS exhibited reduced capillary velocity and sensitivity to reduced PO2. (A) Schematic of the microfluidic setup to measure ex vivo RBC capillary velocity at varied PO2 levels (top). Representative time-lapse images of RBCs from HCs and patients with ME/CFS flowing through a microfluidic capillary at PO2 of 0 mm Hg (bottom; scale bar, 25 μm). (B) The magnitude of capillary velocity and calculated velocity slope (ie, sensitivity) of RBCs from ME/CFS are significantly reduced compared to HCs (HCs, R2 = 0.99; n = 23 participants; 2556 cells; ME/CFS, R2 = 0.92; n = 35 patients; 3847 cells). ns, P > .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001 (Mann-Whitney test). Unpaired t test was used for slope comparison. Each data point in the calculated slope represents the slope from 1 participant. (C) Calculated slope and average RBC velocity (average of 4 PO2) showed no significant dependence on participant age (HCs, n = 23; ME/CFS, n = 35). Each data point represents 1 participant. (D-E) RBC capillary velocity but not the calculated slope (ie, sensitivity) was significantly different between sexes for HCs (D) and ME/CFS (E). Nine healthy male (R2 = 0.93) and 14 healthy female participants (R2 = 1.00) and 10 male (R2 = 0.48) and 25 female (R2 = 0.93) patients with ME/CFS were included. Each data point in the calculated slope represents 1 participant. Data are presented as mean ± standard error of the mean (SEM).
RBCs from ME/CFS exhibited reduced capillary velocity and sensitivity to reduced PO2. (A) Schematic of the microfluidic setup to measure ex vivo RBC capillary velocity at varied PO2 levels (top). Representative time-lapse images of RBCs from HCs and patients with ME/CFS flowing through a microfluidic capillary at PO2 of 0 mm Hg (bottom; scale bar, 25 μm). (B) The magnitude of capillary velocity and calculated velocity slope (ie, sensitivity) of RBCs from ME/CFS are significantly reduced compared to HCs (HCs, R2 = 0.99; n = 23 participants; 2556 cells; ME/CFS, R2 = 0.92; n = 35 patients; 3847 cells). ns, P > .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001 (Mann-Whitney test). Unpaired t test was used for slope comparison. Each data point in the calculated slope represents the slope from 1 participant. (C) Calculated slope and average RBC velocity (average of 4 PO2) showed no significant dependence on participant age (HCs, n = 23; ME/CFS, n = 35). Each data point represents 1 participant. (D-E) RBC capillary velocity but not the calculated slope (ie, sensitivity) was significantly different between sexes for HCs (D) and ME/CFS (E). Nine healthy male (R2 = 0.93) and 14 healthy female participants (R2 = 1.00) and 10 male (R2 = 0.48) and 25 female (R2 = 0.93) patients with ME/CFS were included. Each data point in the calculated slope represents 1 participant. Data are presented as mean ± standard error of the mean (SEM).
The results demonstrated that RBC velocity increased in response to decreasing PO2 for both HCs and individuals with ME/CFS (Figure 1B), aligning with findings from mouse studies.25,26 However, at low PO2 levels (0 and 12 mm Hg), ME/CFS RBC velocities were significantly lower than those of HCs, whereas at elevated PO2 levels (eg, 25 and 34 mm Hg), ME/CFS RBC velocities were comparable to or even exceeded those of HCs. A linear correlation between RBC velocity and PO2 was observed for both HCs (R2 = 0.99) and patients with ME/CFS (R2 = 0.92). The RBC velocity slope, representing the sensitivity of RBC velocity to changes in PO2, was significantly smaller in ME/CFS than HCs (Figure 1B), indicating that RBCs from patients with ME/CFS were less sensitive to local PO2 changes. These data demonstrate that PO2-regulated RBC capillary velocity is impaired in ME/CFS, with a reduced magnitude of capillary velocity at low PO2 and dampened sensitivity to PO2 changes.
We further examined whether the participants’ age and sex affect the magnitude and calculated slope of PO2-regulated RBC capillary velocity. When the calculated slopes were plotted against participants’ age, small correlation coefficients (R2) were observed for both HCs and patients with ME/CFS (for HCs, R2 = 0.042; for ME/CFS, R2 = 0.00005; Figure 1C), indicating a weak linear correlation between the calculated slopes and participants’ age. The correlation coefficient in ME/CFS was 3 orders of magnitude lower than that in HCs. The magnitude of RBC velocity exhibited a similar change with age, in which a small R2 was observed for both HCs (R2 = 0.040) and patients with ME/CFS (R2 = 0.059). No significant difference was observed in terms of fitting slopes between HCs and ME/CFS (for velocity, P = .32; for the calculated slope, P = .52). On the contrary, capillary velocity of RBCs from female participants was significantly higher than that from male participants for both HCs and ME/CFS (Figure 1D-E). The calculated slopes between male and female participants were not significantly different, suggesting that sex affects the magnitude of RBC capillary velocity but not RBC sensitivity to local PO2 changes. Within the same sex, calculated slopes in HCs were still significantly higher than that in ME/CFS (for female, P < .001; for male, P < .01; supplemental Figure 1). These results indicate that, in the current cohort, participants’ age has nonsignificant effects on RBC velocity, whereas sex affects the magnitude but not the calculated slope of PO2-regulated RBC capillary velocity.
Velocity slope–based ROC analysis classifies ME/CFS from HCs but not ME/CFS severity
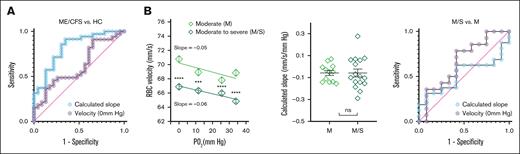
To evaluate the diagnostic performance of our metric in distinguishing patients with ME/CFS from HCs, we generated receiver operator characteristic (ROC) curves using the absolute RBC velocity under hypoxia (0 mm Hg) and the calculated slope of RBC velocity (Figure 2A). ROC analysis yielded an area under the curve (AUC) of 0.62 (95% confidence interval [CI], 0.47-0.77; P > .1) for velocity-based calculation and 0.82 (95% CI, 0.71-0.93; P < .0001) for calculated slope–based calculation. Optimal sensitivity and specificity were achieved with slope thresholds between –0.142 mm/s per mm Hg (sensitivity = 86%; specificity = 70%) and –0.069 mm/s per mm Hg (sensitivity = 57%; specificity = 87%). These findings suggest that the slope of RBC velocity is a more robust biomarker than absolute velocity alone.
ROC analysis of the calculated slope and RBC capillary velocity in classifying ME/CFS. (A) ROC curves of the calculated slope and RBC capillary velocity (at 0 mm Hg) showed AUC values of 0.82 and 0.62, respectively. (B) Capillary velocity of RBCs from M ME/CFS were higher than that from M/S participants with ME/CFS. ∗∗∗P < .001; ∗∗∗∗P < .0001 (Mann-Whitney test). Calculated slope showed no significant difference between the M and M/S groups. ns, P > .05 (Mann-Whitney test; for M, n = 12; for M/S, n = 16). ROC AUC values for calculated slope and RBC capillary velocity (at 0 mm Hg) are 0.52 and 0.66, respectively.
ROC analysis of the calculated slope and RBC capillary velocity in classifying ME/CFS. (A) ROC curves of the calculated slope and RBC capillary velocity (at 0 mm Hg) showed AUC values of 0.82 and 0.62, respectively. (B) Capillary velocity of RBCs from M ME/CFS were higher than that from M/S participants with ME/CFS. ∗∗∗P < .001; ∗∗∗∗P < .0001 (Mann-Whitney test). Calculated slope showed no significant difference between the M and M/S groups. ns, P > .05 (Mann-Whitney test; for M, n = 12; for M/S, n = 16). ROC AUC values for calculated slope and RBC capillary velocity (at 0 mm Hg) are 0.52 and 0.66, respectively.
We further examined whether the severity of ME/CFS affected PO2-regulated RBC capillary velocity and plotted RBC velocity based on severity scored of participants with ME/CFS, using Bell's disability scale ranging from moderate (M) to moderate-to-severe (M/S).46 The results showed that the M group (ie, aged 35-68 years; 10 females and 2 males) exhibited a higher magnitude of RBC velocity than the M/S group (ie, aged 36-68 years; 10 females and 6 males; Figure 2B). The calculated slopes of the M and M/S groups were not significantly different. Furthermore, ROC curve analysis showed an AUC of 0.66 (95% CI, 0.44-0.88; P > .1) and 0.52 (95% CI, 0.30-0.74; P > .5) for velocity slope– and calculated slope–based calculations, respectively, indicating limited significant discriminative power, consistent with the observed lack of statistical difference between the 2 severity groups.
Slope features of PO2-regulated RBC capillary velocity exhibit high accuracy for ME/CFS classification
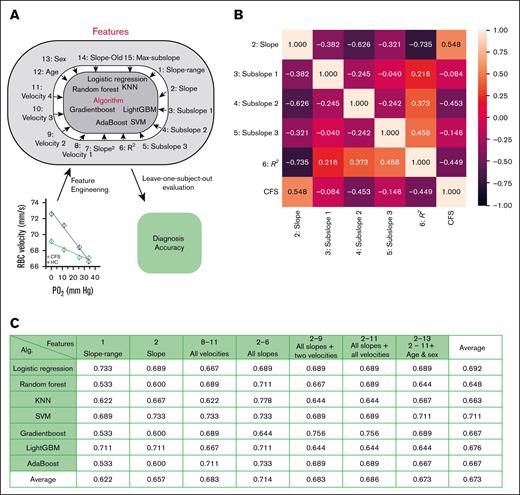
We further evaluated different informative features of PO2-regulated RBC capillary velocity as a potential biomarker for ME/CFS classification. In addition to RBC velocity and slope, these informative features could also include participants’ sex or age, mean RBC velocity at different PO2 levels, subslopes (slopes based on 2 adjacent PO2 levels), and the maximum subslope. Using feature selection techniques and domain knowledge, a range of machine language (ML) algorithms can be trained to identify a specific feature or a combination of features as a classifier that exhibits the highest accuracy, sensitivity, and specificity for ME/CFS classification (Figure 3A)
ML-guided analysis of PO2-regulated RBC capillary velocity for ME/CFS classification. (A) Flow diagram of ML-guided analysis. Various physical parameters or features from PO2-regulated RBC capillary velocity were used as input for different ML algorithms to calculate ME/CFS correlation and diagnostic accuracy. Slope was calculated based on RBC velocities at 4 PO2 levels, whereas slope-range was calculated based on RBC velocity within a range from 25% to 75% of the maximum velocity. Subslopes 1 to 3 were calculated based on velocities of 2 adjacent PO2 levels. R2 was the coefficient of determination calculated based on RBC velocities at 4 PO2 levels. Velocities 1 to 4 represented RBC velocities at PO2 levels of 34, 25, 12, and 0 mm Hg, respectively. Slope-old represented the slope calculated based on the velocities of RBCs falling within the lowest 50% of the velocity range. Maximum subslope was the maximum value of all the subslopes. (B) Correlation heat map between features and ME/CFS. Slope showed the highest correlation (0.548) for ME/CFS classification. (C) Classification accuracy using different subsets of the features and ML algorithms. Combination of features 2 to 6 showed the highest accuracy in KNN (0.778). Calculation was conducted based on data collected from 23 healthy participants and 35 patients with ME/CFS.
ML-guided analysis of PO2-regulated RBC capillary velocity for ME/CFS classification. (A) Flow diagram of ML-guided analysis. Various physical parameters or features from PO2-regulated RBC capillary velocity were used as input for different ML algorithms to calculate ME/CFS correlation and diagnostic accuracy. Slope was calculated based on RBC velocities at 4 PO2 levels, whereas slope-range was calculated based on RBC velocity within a range from 25% to 75% of the maximum velocity. Subslopes 1 to 3 were calculated based on velocities of 2 adjacent PO2 levels. R2 was the coefficient of determination calculated based on RBC velocities at 4 PO2 levels. Velocities 1 to 4 represented RBC velocities at PO2 levels of 34, 25, 12, and 0 mm Hg, respectively. Slope-old represented the slope calculated based on the velocities of RBCs falling within the lowest 50% of the velocity range. Maximum subslope was the maximum value of all the subslopes. (B) Correlation heat map between features and ME/CFS. Slope showed the highest correlation (0.548) for ME/CFS classification. (C) Classification accuracy using different subsets of the features and ML algorithms. Combination of features 2 to 6 showed the highest accuracy in KNN (0.778). Calculation was conducted based on data collected from 23 healthy participants and 35 patients with ME/CFS.
To identify such a classifier, we first computed the correlation between these informative features and ME/CFS. The calculated RBC velocity slope exhibited the strongest correlation with ME/CFS (Figure 3B), whereas other slope-related features, such as slope-range, (slope)2, slope-young, and slope-old, demonstrated high to close-to-highest correlations (supplemental Figure 2A). The magnitudes of velocity at various PO2 levels did not exhibit comparable correlations with ME/CFS as the aforementioned features, and age and sex showed no significant correlation with ME/CFS (supplemental Figure 2A). We then proceeded to train algorithms using ≥1 feature with high correlations to ME/CFS, choosing 7 ML algorithms that are commonly used for classification: logistic regression, random forest, k-nearest neighbors (KNN), support vector machine (SVM), gradient boost, LightGBM, and AdaBoost. We assessed the accuracy, sensitivity, and specificity of these algorithms using the LOSO evaluation methodology, an established approach in classification.47 For each algorithm and feature set combination, all participants except 1 were used for training, and then the left-out participant was used for testing. LOSO simulates evaluation on an independent data set by leaving each participant out of training, allowing validation on unseen data and ensuring robust performance across individuals. This process was iteratively applied to each participant and the overall accuracy, sensitivity, and specificity derived from the average results of all iterations.
Through these evaluations, we demonstrated that the combination of all slope features, that is, features 2 to 6, exhibited the highest average accuracy (71.4%), sensitivity (70.3%), and specificity (70.8%) among all the informative features tested (Figure 3C; supplemental Figure 2B-C). Features 2 to 6 exhibited an accuracy of 77.8% in KNN, a sensitivity of 76% in AdaBoost, and a specificity of 90% in SVM, indicating that these features may contain the most predictive information for this classification task. Meanwhile, across multiple feature sets, SVM showed the highest average accuracy (71.1%) and specificity (79%), whereas LightGBM had the best sensitivity results (70.9%). KNN was the only algorithm that produced accuracy (77.8%), sensitivity (75.8%), and specificity (81.2%) all >75%. Other combinations of algorithm and feature set combinations, feature selection algorithms, and ensemble learning were also experimented with but generated no significant differences and thus were not reported here. Collectively, these results demonstrate that the combination of slope features in PO2-regulated RBC capillary velocity exhibits the highest accuracy to differentiate ME/CFS from HCs.
Salmeterol and xanomeline treatments improve PO2-regulated RBC capillary velocity in ME/CFS
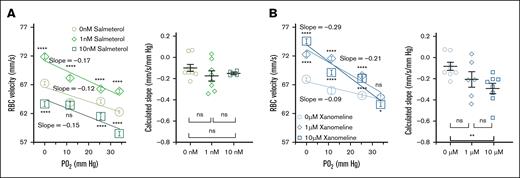
Because RBC membrane deformability played a critical role in PO2-regulated RBC capillary velocity,26 we hypothesized that drugs that can increase RBC membrane deformability could improve PO2-regulated RBC capillary velocity in ME/CFS.16,18 To test this hypothesis, we treated RBCs from participants with ME/CFS with salmeterol xinafoate or xanomeline and measured RBC velocity accordingly. Salmeterol xinafoate and xanomeline, used to treat asthma48 and schizophrenia,49 respectively, are known to increase RBC deformability.50-53 We isolated RBCs from 9 participants with ME/CFS and incubated them with 0, 1, or 10 nM salmeterol xinafoate (which was first dissolved in DMSO) in PBS at room temperature for 15 minutes. The treated RBCs were then injected into the microfluidic capillary to measure RBC capillary velocity at reduced PO2 levels. As depicted in Figure 4A, RBCs treated with 1 nM salmeterol exhibited the highest capillary velocity, whereas RBCs treated with 10 nM salmeterol had the lowest velocity. The slope calculated from each participant was not significantly different between salmeterol treatments but trended toward a higher slope in 1 nM salmeterol treatment. Linearity analysis showed a similar trend, with the highest R2 for 1 nM salmeterol treatment (R2 = 0.81, 0.91, and 0.85 for 0, 1, and 10 nM salmeterol, respectively). These data demonstrate that salmeterol treatments improve the magnitude of RBC capillary velocity in a dose-dependent manner but do not significantly affect RBC velocity slope.
Salmeterol and xanomeline improved PO2-regulated RBC capillary velocity and sensitivity in ME/CFS. (A) RBCs treated with 1 nM salmeterol exhibited the highest magnitude and sensitivity of PO2-regulated capillary velocity (patients with ME/CFS, n = 9; 955, 956, and 473 cells for 0 nM (R2 = 0.81), 1 nM (R2 = 0.91) and 10 nM salmeterol (R2 = 0.85), respectively. Calculated slope of –0.098 ± 0.0331, –0.175 ± 0.0485, and –0.149 ± 0.0121 mm/s per mm Hg for 0, 1, and 10 nM salmeterol, respectively. ns, P > .05 (Mann-Whitney test). (B) Xanomeline incubation increased the magnitude and sensitivity of PO2-regulated RBC capillary velocity (patients with ME/CFS, n = 8; 905, 690, and 917 cells for 0-μM (R2 = 0.92), 1-μM (R2 = 0.91), and 10-μM xanomeline (R2 = 0.91), respectively. Calculated slope (mm/s per mm Hg) of –0.083 ± 0.0381, –0.204 ± 0.0730, and –0.292 ± 0.0478 for 0, 1, and 10 μM, respectively. ns, P > .05 (Mann-Whitney test); ∗∗P < .01 (unpaired t test). Each data point in calculated slope represents 1 participant. Data are presented as mean ± SEM.
Salmeterol and xanomeline improved PO2-regulated RBC capillary velocity and sensitivity in ME/CFS. (A) RBCs treated with 1 nM salmeterol exhibited the highest magnitude and sensitivity of PO2-regulated capillary velocity (patients with ME/CFS, n = 9; 955, 956, and 473 cells for 0 nM (R2 = 0.81), 1 nM (R2 = 0.91) and 10 nM salmeterol (R2 = 0.85), respectively. Calculated slope of –0.098 ± 0.0331, –0.175 ± 0.0485, and –0.149 ± 0.0121 mm/s per mm Hg for 0, 1, and 10 nM salmeterol, respectively. ns, P > .05 (Mann-Whitney test). (B) Xanomeline incubation increased the magnitude and sensitivity of PO2-regulated RBC capillary velocity (patients with ME/CFS, n = 8; 905, 690, and 917 cells for 0-μM (R2 = 0.92), 1-μM (R2 = 0.91), and 10-μM xanomeline (R2 = 0.91), respectively. Calculated slope (mm/s per mm Hg) of –0.083 ± 0.0381, –0.204 ± 0.0730, and –0.292 ± 0.0478 for 0, 1, and 10 μM, respectively. ns, P > .05 (Mann-Whitney test); ∗∗P < .01 (unpaired t test). Each data point in calculated slope represents 1 participant. Data are presented as mean ± SEM.
Similarly, we incubated RBCs from another 8 participants with ME/CFS with 0-, 1-, or 10-μM xanomeline and measured RBC capillary velocity after treatment. Given that a previous study demonstrated a significant response in Chinese hamster ovary cells with 1-μM xanomeline,54 we chose to treat RBCs with 1- or 10-μM xanomeline. Compared to the 0-μM xanomeline treatment, RBCs treated with 1- and 10-μM xanomeline exhibited increased capillary velocity with increasing xanomeline concentration (Figure 4B). RBCs treated with 10-μM xanomeline had the highest capillary velocity, whereas RBCs treated with 0-μM xanomeline had the lowest velocity. The linear relations between RBC velocity and PO2 were not significantly different among the xanomeline treatments (R2 = 0.92, 0.91, and 0.91 for 0-, 1-, and 10-μM xanomeline, respectively). The slope calculated from each participant increased with increasing xanomeline concentration and exhibited a significant increase between 0- and 10-μM xanomeline treatments. These results demonstrate that xanomeline treatments improve both the magnitude of RBC capillary velocity and RBC velocity slope in a dose-dependent manner. Incubation of RBCs from a HC with salmeterol or xanomeline showed similar trends as observed in ME/CFS, except that the RBC velocity was higher in the HC (supplemental Figure 3A-B), consistent with the results in Figure 1. DMSO had neglectable effects on PO2-regulated RBC capillary velocity in both HC and ME/CFS (supplemental Figure 3C-D).
Discussion
ME/CFS is a complex disease affecting multiple cerebral processes, including cerebral hypoperfusion and cognitive dysfunctions. The underlying mechanisms of impaired CBF in ME/CFS remain unclear and are likely caused by a combination of factors.55 The impaired PO2-regulated RBC velocity in ME/CFS observed here suggests compromised oxygen delivery, aligning with previous studies showing altered blood flow patterns, prolonged recovery times for oxygen saturation, and tissue hypoxia.56,57 The impaired RBC responses to changes in PO2 (ie, calculated RBC velocity slopes) may result from ME/CFS-induced cellular and molecular abnormality in RBCs. For example, reduced numbers of discoid RBCs,58,59 increased RBC distribution width,60 and reduced RBC deformability61 have been observed in ME/CFS. Such cellular impairments in RBCs are known to negatively affect RBC capillary flow62 and thus likely contribute to the impaired RBC responses to low PO2 in ME/CFS. Furthermore, the amount of 2,3-diphosphoglyceric acid and oxidized hemoglobin (ie, methemoglobin) in RBCs is increased in patients with ME/CFS.27,63 Elevated levels of RBC 2,3-diphosphoglyceric acid attenuate PO2-regulated RBC capillary velocity,26 and increased methemoglobin reduces the amount of unoxidized hemoglobin available for hemoglobin–band 3 interaction at reduced PO2, which may underlie the molecular characteristics of dampened RBC responses to PO2 changes in ME/CFS.
Our findings also indicate that capillary velocities of RBCs derived from female cohorts (both HCs and patients with ME/CFS) are higher than those from male cohorts, consistent with prior research showing increased RBC deformability in females.64 Female participants also typically displayed a lower mean red cell volume than male participants.65 The smaller RBC volume in female participants could facilitate RBC transport across the constriction channel, supporting our data in Figure 1D-E. Although statistical analyses yield a smaller P value for female participants (supplemental Figure 1; for females, P < .001; for males, P < .01), suggesting a potentially more pronounced compromise among female participants, average calculated slopes do not demonstrate a significant difference between male and female participants for both ME/CFS and HCs.
Velocity slope–based ROC analysis yielded a decent AUC of 0.82, which is comparable or higher than that of recent studies in ME/CFS diagnosis. Eguchi et al demonstrated that circulating extracellular vehicles (EVs) containing actin-network proteins, specifically talin-1 and filamin-A, showed promise as blood-based biomarkers for ME/CFS.66 EV counts alone yielded an AUC of 0.80 in the ROC analysis. This result supports the view that noninvasive, liquid biopsy–based markers, particularly those reflecting cytoskeletal or structural cell changes, can effectively distinguish patients with ME/CFS from healthy individuals. In another study, circulating microRNA signatures yielded an AUC of 0.65 when distinguishing participants with ME/CFS from HCs (sensitivity = 70%; specificity = 60%).67 Sato et al evaluated specific immunoglobulin heavy variable genes as diagnostic markers for ME/CFS and reported an AUC of 0.907 in cohort 1 and 0.846 in cohort 2.68 The 2-day cardiopulmonary exercise test, a commonly used functional marker, yielded an AUC value of ∼0.88 when analyzing postexertional drops in ventilatory threshold.69 Notably, we observed that absolute RBC velocity under hypoxia (0 mm Hg) yielded a lower AUC (0.62; P > .1), reinforcing the concept that stress-induced responses, rather than static measurements, provide more sensitive and specific diagnostic insights in ME/CFS. This supports the emerging idea that dynamic physiological perturbations, such as hypoxia or exertion, are necessary to reveal subtle pathophysiological dysfunction characteristics of ME/CFS.
The ML approach further confirms the ROC analysis, demonstrating that slope-based RBC features are of the highest accuracy (77.8%), sensitivity (76%), and specificity (90%) for ME/CFS classification among 7 classic classification algorisms. Alternations in RBC shape and deformability under capillary flow have been observed in diseases such as COVID-19, suggesting that examining RBC shape changes in capillary flow could aid diagnosis in other conditions.70,71 For ME/CFS diagnosis, a recent study by Xu et al72,73 measured Raman spectral data of peripheral blood mononuclear cells (PBMCs) from patients with ME/CFS and trained the data using an ensemble of multiple classification algorithms (ie, ensemble learning). The classification accuracy reported in Xu et al was ∼91%. However, unlike the LOSO evaluation methodology used here, the training and testing data sets in Xu et al’s study73 were derived from the same pool of participants, potentially leading to information leakage and inflated accuracy. Another related study by Esfandyarpour et al developed a novel nanoelectronic assay to measure impedance of PBMCs in patients with ME/CFS and demonstrated that impedance patterns from PBMCs could be used to train a SVM to establish a classifier for ME/CFS.74 However, they did not report classification accuracy in that study. The ML techniques demonstrated here analyzed novel RBC velocity features and offered reliable and robust data characterization that extends beyond classification accuracy.
Lastly, we showed that salmeterol xinafoate and xanomeline treatments improved PO2-regulated RBC capillary velocity in ME/CFS. Previous studies have demonstrated that stimulating the adenylyl cyclase–cAMP–protein kinase A pathway, either via β-adrenergic receptors or direct activation, increases RBC deformability. Exposure of human RBCs to β-adrenergic agonists such as adrenaline (epinephrine) and isoproterenol resulted in up to 45% increase in the maximal amplitude of cell membrane fluctuations, measured by time-dependent light scattering microscopy (ie, point dark field microscopy), as well as shorter transmit time through a polycarbonate filter (a pore size of 5 μm), suggesting increased RBC deformability.75,76 This effect was amplified under hypoxic conditions and mediated through a cAMP-dependent pathway. In addition, human RBCs treated with forskolin (direct adenylyl cyclase activator) exhibited increased RBC deformability measured by RBC elongation index using laser ektacytometry.76 These effects are modulated by shear stress, demonstrating that β-adrenergic stimulation affects RBC properties under physiological flow conditions. Furthermore, RBCs treated with pentoxifylline, a hemorheological agent, exhibited increased deformability.77 Pentoxifylline is a phosphodiesterase inhibitor and leads to increased intracellular cAMP. IV injection of pentoxifylline (1200-1500 mg/d) in patients for 10 days led to a marked decrease in RBC transmit time through an ex vivo 5-μm pore filtration system, indicating improved RBC deformability. Salmeterol xinafoate is a long-acting β2-adrenergic agonist that acts on β2 receptors on the RBC membrane, resulting in elevated cAMP levels in RBCs53 and increased RBC deformability,75 which is expected to lead to an increased capillary velocity.78 RBCs treated with β-adrenergic agonists, that is, isoproterenol,75 were known to exhibit a bell-shaped, dose-dependent cell membrane fluctuation, in which RBC deformability increased monotonically when 0.1 to 10 nM β-adrenergic agonists were used. Although the concentration of salmeterol xinafoate used here (1 nM and 10 nM) fell into this concentration range, PO2-regulated RBC capillary velocity increased in 1 nM but not in 10 nM salmeterol-treated RBCs. This discrepancy is likely because isoproterenol is a nonselective β receptor agonist, whereas salmeterol is a β2-adrenergic agonist. The bell-shaped, dose-dependent RBC cell membrane fluctuation may shift to a lower concentration when salmeterol was used to treat RBC.
Xanomeline, a muscarinic agonist, activates muscarinic receptors on RBCs, leading to increased cGMP production52 and RBC deformability.51 RBCs express muscarinic acetylcholine (ACh) receptors, particularly the M1 and M3 subtypes, and activation of these receptors has been associated with increased RBC deformability and decreased aggregation. For instance, ACh, a key neurotransmitter that activates muscarinic receptors, can bind to RBCs, increasing RBC deformability and reducing RBC aggregation.51 In this case, the ACh-muscarinic receptor interaction triggers G-protein signaling, altering downstream protein kinase A activity (eg, band 3 phosphorylation) and enhancing RBC deformability. Thus, incubating RBCs with xanomeline potentially initiates a cascade of events, leading to the phosphorylation of tyrosine residues on band 3 protein within RBCs,50 resulting in the detachment of band 3 from its ankyrin tethering on the spectrin-actin cytoskeleton,26 and, consequently, increasing RBC deformability. Because RBC hemoglobin–band 3 interaction regulates PO2-regulated RBC capillary velocity, the effects of xanomeline on band 3 phosphorylation may explain the improved responsiveness of RBC to reduced PO2. Such improved RBC capillary velocity upon salmeterol and xanomeline treatments may provide new pharmaceutical approaches to rescue impaired blood flow in ME/CFS.16,18,56
In summary, we demonstrated that RBCs from individuals with ME/CFS exhibited significantly impaired capillary velocity at reduced PO2 ex vivo using microfluidics. This PO2-regulated RBC capillary velocity provides a new and effective approach for ME/CFS classification, monitoring, and assessment. Furthermore, RBCs treated with salmeterol and xanomeline showed improved capillary velocity and responsiveness to reduced PO2 in ME/CFS. Given the essential role of RBCs in maintaining physiological function, RBC dysfunction or altered biomechanics may significantly contribute to blood flow abnormalities observed in ME/CFS. We acknowledge the limitations of this study, which is being continued with a larger patient cohort while simultaneously improving detection accuracy, sensitivity, and ease of use. Despite the limited sample size of this study, this RBC-based microfluidic approach interrogates RBC responses to reduced PO2, revealing previously unrealized roles of RBCs in pathophysiology of ME/CFS and offering a novel approach toward ME/CFS diagnosis.
Acknowledgments
The authors thank Anna Okumu for participant recruitment and Amrit Shahzad for helpful discussions.
This study was supported by a National Institute of Health grant 1R21AI175960-01A (J.W.) and by the Open Medicine Foundation (A22-2417; J.W.).
Authorship
Contribution: J.W., R.W.D., and Y.G. designed research; J.W., Y.G., X.L., M.N.-G., S.Z., and M.G. wrote the manuscript; Y.G. and S.Z. performed ex vivo microfluidic experiments; S.R., X.L., and Y.G. performed machine learning experiments; and Y.G., J.W., S.R., X.L., and S.Z. conducted data analysis and calculation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jiandi Wan, Department of Chemical Engineering, University of California Davis, CA 95616; email: jdwan@ucdavis.edu.
References
Author notes
Data are available on request from the corresponding author, Jiandi Wan (jdwan@ucdavis.edu). The manuscript will also be shared in public deposit according to the National Institutes of Health policy.
The full-text version of this article contains a data supplement.