Key Points
Asciminib is an effective option in later lines of CML therapy, particularly in patients with previous intolerance to other TKIs.
There was a low rate of cardiovascular events; grade 3 and 4 thrombocytopenia and neutropenia occurred in 26% and 17%, respectively.
Visual Abstract
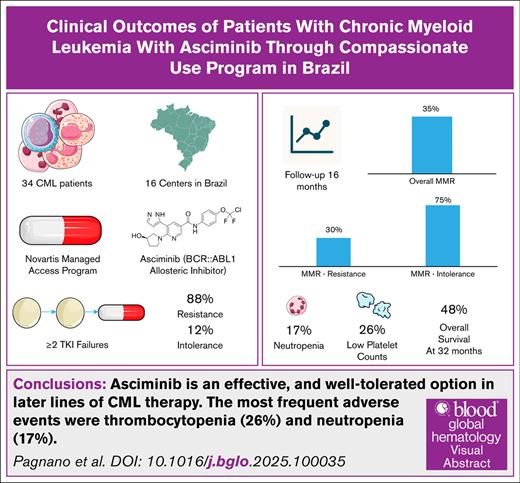
This study evaluated the clinical outcomes of patients with chronic myeloid leukemia (CML) treated with asciminib, a first-in-class allosteric inhibitor that selectively targets the ABL myristoyl pocket (STAMP) of BCR::ABL1. Through Novartis’s Managed Access Program in Brazil, asciminib was provided to patients with resistance or intolerance to ≥2 tyrosine kinase inhibitors (TKIs). Among 34 patients included, 30 (88.2%) had resistance to prior TKIs and 4 (11.8%) had intolerance; 7 patients carried the T315I mutation. After a median follow-up of 14 months, 35% of the cohort achieved a major molecular response (MMR). Only 1 patient with the T315I mutation reached MMR. Patients intolerant to previous TKIs responded more favorably than those with resistance (75% vs 30%; P = .07). The most frequent adverse events were thrombocytopenia (26%) and neutropenia (17%); 1 patient experienced an acute myocardial infarction. Six patients discontinued treatment, including 4 due to blast crisis or sepsis (n = 2). Two unplanned pregnancies led to temporary treatment interruption, with both patients regaining MMR after the reintroduction of asciminib postdelivery. At 32 months, the overall survival rate was 48%. These findings indicate that asciminib is a well-tolerated and effective therapeutic option for patients with CML in later lines of treatment.
Introduction
Asciminib is a novel, first-in-class allosteric inhibitor that selectively targets the ABL myristoyl pocket (STAMP) of BCR::ABL1, locking the kinase in an inactive conformation.1,2 Asciminib has been approved for the treatment of patients with chronic myeloid leukemia (CML) in chronic phase (CP) previously treated with ≥2 tyrosine kinase inhibitors (TKIs), based on results from the pivotal ASCEMBL trial,3,4 in which it was compared with bosutinib. Asciminib has also demonstrated efficacy against the T315I mutation in the phase 1 X2101 trial.5 To evaluate its real-world performance, several Managed Access Programs (MAPs) have been implemented worldwide, providing access to asciminib before regulatory approval.6-12 Here, we report the real-world clinical outcomes of Brazilian patients with CML treated with asciminib provided through Novartis’s MAP after resistance or intolerance to ≥2 TKIs. We aimed to evaluate the clinical outcomes of patients with CML treated with asciminib provided through Novartis’s MAP in Brazil.
Methods
Hematologists who had requested asciminib through the MAP in Brazil were approached to contribute data on the clinical outcomes of their patients with CML. We collected demographic data, CML and medical history, previous treatments, responses, and adverse events from patients with CML treated with asciminib. The research project was approved by the University of Campinas Ethics Committee and conducted in accordance with the Declaration of Helsinki. All the patients provided signed informed consent. Disease phase was classified according to the World Health Organization 2016 classification.13 Resistance and response criteria were defined according to the European LeukemiaNet recommendations.14
We defined intolerance as nonhematologic grade 3 or 4 toxicity during therapy; persistent grade 2 toxicity unresponsive to optimal management, including dose adjustments; or hematologic grade 3 or 4 toxicity during therapy that recurred after dose reduction to the lowest recommended dose.4 We graded adverse events using the Common Terminology Criteria for Adverse Events Version 5.0.15
The cutoff date of this analysis was February 2025.
Statistical analyses
All analyses were descriptive, and no statistical comparisons were performed. Overall survival (OS) was estimated using the Kaplan-Meier method from the start of asciminib until death, last follow-up or treatment discontinuation. Given that no deaths occurred during the study period, the discontinuation of asciminib was considered as the event. All analyses were conducted using IBM-SPSS software for Windows 24.
Results
Between August 2018 and March 2024, 34 patients from 16 centers were treated with asciminib provided by the MAP. At diagnosis, the median age of the patients was 44.5 years (range, 6-74); 22 (64.7%) were male, 31 (91.2%) were in the CP, and 3 (8.8%) in the accelerated phase (AP). Seven patients (20.6%) had low Sokal risk, followed by 8 (23.5%) with intermediate, 9 (26.5%) with high, and 10 (29.4%) with unknown risks. Regarding the European Treatment and Outcome Study long-term survival score, 11 (32.40%) were classified as low risk, 11 (32.4%) as intermediate, and 5 (14.77%) as high risk; 7 (20.6%) were classified as unknown (Tables 1 and 2).
Clinical and laboratory characteristics of patients with CML at diagnosis and at asciminib initiation (n = 34)
| Variables . | n (%) . |
|---|---|
| Age at diagnosis, median (range), y | 44.5 (6-74) |
| Age at asciminib initiation, median (range), y | 51.5 (14-78) |
| Sex, male | 22 (64.7) |
| CML phase at diagnosis | |
| Chronic | 31 (91.2) |
| Accelerated | 3 (8.8) |
| CML phase at asciminib initiation | |
| Chronic | 26 (83) |
| Accelerated | 4 (12.5) |
| BC | 2 (6) |
| Sokal score (diagnosis) | |
| Low | 7 (20.6) |
| Intermediate | 8 (23.5) |
| High | 9 (26.5) |
| Missing | 10 (29.4) |
| ELTS score (diagnosis) | |
| Low | 11 (32.4) |
| Intermediate | 14 (43) |
| High | 5 (14.7) |
| Missing | 7 (20.6) |
| BCR::ABL1 mutations | |
| No mutations | 20 (58.8) |
| T315I | 7 (20.5) |
| Other mutations∗ | 6 (18) |
| Not analyzed | 1 (2.9) |
| ACAs at asciminib initiation | |
| No ACAs | 16 (47) |
| Not available | 17 (50) |
| Missing | 1 (3) |
| Baseline BCR::ABL1 transcripts % IS (before asciminib), median (range) | 25 (0.1-184) |
| Previous therapeutics lines | |
| 2-3 | 29 (85.3) |
| ≥4† | 5 (14.7) |
| Variables . | n (%) . |
|---|---|
| Age at diagnosis, median (range), y | 44.5 (6-74) |
| Age at asciminib initiation, median (range), y | 51.5 (14-78) |
| Sex, male | 22 (64.7) |
| CML phase at diagnosis | |
| Chronic | 31 (91.2) |
| Accelerated | 3 (8.8) |
| CML phase at asciminib initiation | |
| Chronic | 26 (83) |
| Accelerated | 4 (12.5) |
| BC | 2 (6) |
| Sokal score (diagnosis) | |
| Low | 7 (20.6) |
| Intermediate | 8 (23.5) |
| High | 9 (26.5) |
| Missing | 10 (29.4) |
| ELTS score (diagnosis) | |
| Low | 11 (32.4) |
| Intermediate | 14 (43) |
| High | 5 (14.7) |
| Missing | 7 (20.6) |
| BCR::ABL1 mutations | |
| No mutations | 20 (58.8) |
| T315I | 7 (20.5) |
| Other mutations∗ | 6 (18) |
| Not analyzed | 1 (2.9) |
| ACAs at asciminib initiation | |
| No ACAs | 16 (47) |
| Not available | 17 (50) |
| Missing | 1 (3) |
| Baseline BCR::ABL1 transcripts % IS (before asciminib), median (range) | 25 (0.1-184) |
| Previous therapeutics lines | |
| 2-3 | 29 (85.3) |
| ≥4† | 5 (14.7) |
ACA, additional cytogenetic abnormality; ELTS, European Treatment and Outcome Study long-term survival; IS, international scale.
E255K mutation, n = 1; E255V/F317L mutation, n = 1; F359V mutation, n = 1; and exon 7 deletion, n = 3.
Two patients had previous HSCT.
Clinical data from patients with CML treated with ASC (n = 34)
| Patient no. . | Age at ASC initiation, y . | Year of CML diagnosis . | Disease phase at diagnosis . | Disease phase at ASC initiation . | Previous treatments . | No. of previous lines . | Mutation before ASC . | Outcome∗ . |
|---|---|---|---|---|---|---|---|---|
| 1 | 77 | 2017 | CP | CP | IM, DASA, and NILO | 3 | 0 | Alive, with CCyR |
| 2 | 55 | 2015 | CP | CP | IM and DASA | 2 | 0 | Alive, with MMR |
| 3 | 44 | 2021 | CP | CP | IM and NILO | 2 | 0 | Alive, MR4.0 |
| 4 | 48 | 1998 | CP | CP | IFN, IM, DASA, NILO, and PONA | 5 | 0 | Alive, CHR |
| 5 | 47 | 2019 | CP | CP | IM, DASA, and NILO | 2 | 0 | Alive, with MMR |
| 6 | 82 | 2020 | CP | AP | IM, DASA, and NILO | 3 | 0 | Alive, with AP |
| 7 | 34 | 2017 | CP | CP | IM and DASA | 2 | 0 | Alive, with MMR |
| 8 | 68 | 2010 | CP | CP | IM, DASA, and NILO | 3 | T315I | Alive, with CHR |
| 9 | 34 | 1994 | CP | CP | IM, DASA, NILO, and HSCT† | 5 | 0 | Alive, with CHR |
| 10 | 63 | 2019 | CP | CP | IM and NILO | 2 | 0 | Alive, with DMR |
| 11 | 35 | 2021 | CP | CP | IM and NILO | 2 | F359V | Alive, on DASA |
| 12 | 16 | 2014 | CP | CP | IM, DASA, and PONA | 3 | E255V/F317L | Alive, with DMR |
| 13 | 56 | 2018 | CP | CP | IM, DASA, NILO, and PONA | 4 | 0 | Alive, with MMR |
| 14 | 40 | 2017 | CP | BC | IM, DASA, and NILO | 3 | 0 | Dead, after HSCT |
| 15 | 51 | 2018 | CP | BC | IM, DASA, NILO, PONA, and HSCT | 5 | T315I | Dead, with BC |
| 16 | 55 | 2021 | CP | CP | IM and DASA | 2 | T315I | Alive, with MMR |
| 17 | 67 | 2022 | CP | CP | IM and DASA | 2 | Exon 7 deletion | Dead, with BC |
| 18 | 73 | 2021 | CP | CP | IM, DASA, and NILO | 3 | Exon 7 deletion | Alive, with CHR |
| 19 | 64 | 2021 | CP | CP | IM and NILO | 2 | Exon 7 deletion | Alive, with CHR |
| 20 | 49 | 2014 | CP | CP | IM, DASA, and NILO | 3 | 0 | Alive, with MMR |
| 21 | 42 | 2000 | CP | CP | IM and DASA | 2 | 0 | Alive, with CHR |
| 22 | 67 | 1998 | AP | CP | IM, DASA, and NILO | 3 | E255K | Alive, with CHR |
| 23 | 74 | 2012 | CP | CP | IM, DASA, and NILO | 3 | 0 | Alive, with DMR |
| 24 | 53 | 2020 | CP | CP | IM, DASA, and PONA | 3 | T315I | Alive, with CHR |
| 25 | 77 | 2007 | CP | CP | IM, DASA, and NILO | 3 | 0 | Alive, with MMR |
| 26 | 72 | 2006 | CP | CP | IM, DASA, and NILO | 3 | 0 | Alive, with MMR |
| 27 | 26 | 2016 | CP | AP | IM, DASA, and NILO | 3 | Not done | Alive, on PONA |
| 28 | 64 | 2017 | CP | CP | IM and DASA | 2 | T315I | Dead, with BC |
| 29 | 83 | 2004 | CP | CP | IM, DASA, and NILO | 3 | 0 | Alive, with CHR |
| 30 | 38 | 2017 | CP | CP | IM, DASA, and NILO | 3 | 0 | Dead, with sepsis |
| 31 | 47 | 2018 | CP | CP | IM, DASA, and NILO | 3 | 0 | Alive, with MMR |
| 32 | 39 | 2012 | CP | CP | IM, DASA, and NILO | 3 | 0 | Alive, after HSCT, on DASA |
| 33 | 78 | 2008 | AP | AP | IM, DASA, and NILO | 6 | T315I | Dead, with BC, on PONA |
| 34 | 72 | 2019 | AP | AP | IM, DASA, and NILO | 3 | T315I | Alive, with MR2 |
| Patient no. . | Age at ASC initiation, y . | Year of CML diagnosis . | Disease phase at diagnosis . | Disease phase at ASC initiation . | Previous treatments . | No. of previous lines . | Mutation before ASC . | Outcome∗ . |
|---|---|---|---|---|---|---|---|---|
| 1 | 77 | 2017 | CP | CP | IM, DASA, and NILO | 3 | 0 | Alive, with CCyR |
| 2 | 55 | 2015 | CP | CP | IM and DASA | 2 | 0 | Alive, with MMR |
| 3 | 44 | 2021 | CP | CP | IM and NILO | 2 | 0 | Alive, MR4.0 |
| 4 | 48 | 1998 | CP | CP | IFN, IM, DASA, NILO, and PONA | 5 | 0 | Alive, CHR |
| 5 | 47 | 2019 | CP | CP | IM, DASA, and NILO | 2 | 0 | Alive, with MMR |
| 6 | 82 | 2020 | CP | AP | IM, DASA, and NILO | 3 | 0 | Alive, with AP |
| 7 | 34 | 2017 | CP | CP | IM and DASA | 2 | 0 | Alive, with MMR |
| 8 | 68 | 2010 | CP | CP | IM, DASA, and NILO | 3 | T315I | Alive, with CHR |
| 9 | 34 | 1994 | CP | CP | IM, DASA, NILO, and HSCT† | 5 | 0 | Alive, with CHR |
| 10 | 63 | 2019 | CP | CP | IM and NILO | 2 | 0 | Alive, with DMR |
| 11 | 35 | 2021 | CP | CP | IM and NILO | 2 | F359V | Alive, on DASA |
| 12 | 16 | 2014 | CP | CP | IM, DASA, and PONA | 3 | E255V/F317L | Alive, with DMR |
| 13 | 56 | 2018 | CP | CP | IM, DASA, NILO, and PONA | 4 | 0 | Alive, with MMR |
| 14 | 40 | 2017 | CP | BC | IM, DASA, and NILO | 3 | 0 | Dead, after HSCT |
| 15 | 51 | 2018 | CP | BC | IM, DASA, NILO, PONA, and HSCT | 5 | T315I | Dead, with BC |
| 16 | 55 | 2021 | CP | CP | IM and DASA | 2 | T315I | Alive, with MMR |
| 17 | 67 | 2022 | CP | CP | IM and DASA | 2 | Exon 7 deletion | Dead, with BC |
| 18 | 73 | 2021 | CP | CP | IM, DASA, and NILO | 3 | Exon 7 deletion | Alive, with CHR |
| 19 | 64 | 2021 | CP | CP | IM and NILO | 2 | Exon 7 deletion | Alive, with CHR |
| 20 | 49 | 2014 | CP | CP | IM, DASA, and NILO | 3 | 0 | Alive, with MMR |
| 21 | 42 | 2000 | CP | CP | IM and DASA | 2 | 0 | Alive, with CHR |
| 22 | 67 | 1998 | AP | CP | IM, DASA, and NILO | 3 | E255K | Alive, with CHR |
| 23 | 74 | 2012 | CP | CP | IM, DASA, and NILO | 3 | 0 | Alive, with DMR |
| 24 | 53 | 2020 | CP | CP | IM, DASA, and PONA | 3 | T315I | Alive, with CHR |
| 25 | 77 | 2007 | CP | CP | IM, DASA, and NILO | 3 | 0 | Alive, with MMR |
| 26 | 72 | 2006 | CP | CP | IM, DASA, and NILO | 3 | 0 | Alive, with MMR |
| 27 | 26 | 2016 | CP | AP | IM, DASA, and NILO | 3 | Not done | Alive, on PONA |
| 28 | 64 | 2017 | CP | CP | IM and DASA | 2 | T315I | Dead, with BC |
| 29 | 83 | 2004 | CP | CP | IM, DASA, and NILO | 3 | 0 | Alive, with CHR |
| 30 | 38 | 2017 | CP | CP | IM, DASA, and NILO | 3 | 0 | Dead, with sepsis |
| 31 | 47 | 2018 | CP | CP | IM, DASA, and NILO | 3 | 0 | Alive, with MMR |
| 32 | 39 | 2012 | CP | CP | IM, DASA, and NILO | 3 | 0 | Alive, after HSCT, on DASA |
| 33 | 78 | 2008 | AP | AP | IM, DASA, and NILO | 6 | T315I | Dead, with BC, on PONA |
| 34 | 72 | 2019 | AP | AP | IM, DASA, and NILO | 3 | T315I | Alive, with MR2 |
ASC, asciminib; CCyR, complete cytogenetic response; DASA, dasatinib; DMR, deep molecular response; IFN, interferon; IM, imatinibe; NILO, nilotinib; PONA, ponatinib.
If a patient discontinued ASC, the new drug is cited.
The patient underwent 2 transplants.
All patients received imatinib as first-line treatment. Eleven patients (32.3%) received 2 lines of therapy before asciminib, 18 (53%) received 3 lines, and 5 (14.7%) received ≥4 lines, including 2 patients with previous hematopoietic stem cell transplantation (HSCT). Patients’ characteristics and outcomes are described in Tables 1 and 2.
Two patients received asciminib in clinical trials before entering the program. One patient was previously treated with asciminib in a clinical trial from December 2018 to May 2019. The drug was discontinued due to resistance, and the T315I mutation was subsequently detected. Asciminib was reintroduced through the MAP 1 year later, in March 2020, at a dose of 200 mg twice daily. This restart date was used for the current analysis.
The median time from imatinib initiation to asciminib start was 63.5 months (range, 17-267). Overall, 71 patients (61.7%) had hematologic relapse before starting asciminib. At asciminib initiation, the median age was 51.5 years (range, 14-78); 28 (83%) were in CP, 4 (12.5%) were in AP, and 2 (6%) were in blast crisis (BC).
Resistance was the most frequent indication for using asciminib (n = 30 [88.2%]), whereas 4 patients (11.8%) switched to asciminib due to intolerance to the previous TKI. Before asciminib treatment, 13 patients (38%) had BCR::ABL1 mutations, including 7 (20.6%) with T315I mutation, 1 (2.9%) with E255K mutation, 1 (2.9%) with E255V/F317L mutation, 1 (2.9%) with F359V mutation, and 3 (8.8%) with exon 7 deletion. Mutations were not detected in 20 patients (58.8%) and were not analyzed in 1 (2.9%).
Most patients were treated with asciminib at 80 mg once daily (n = 17 [50%]) or 40 mg twice daily (n = 10 [28%]), whereas 1 patient received asciminib at 20 mg twice daily (2.9%). Patients with the T315I mutation received asciminib 200 mg twice daily (n = 7). Twenty patients (58.8%) had prior comorbidities, including 9 with hypertension, 2 with cardiopathy, 2 with previous acute myocardial infarction, 2 with diabetes, and 1 each with pulmonary hypertension, dyslipidemia, peripheral artery disease, anterior ischemic optic neuropathy, and vascular cerebral stroke.
Two patients temporarily interrupted asciminib during the program because of nonplanned pregnancies. One patient had a twin pregnancy, recently reported by Gutierrez et al16. Both patients restarted treatment after delivery and recovered major molecular response (MMR) after asciminib reintroduction.
Molecular response (MR) before asciminib initiation showed that 23 patients had a baseline quantitative reverse transcription polymerase chain reaction >10% (67%), 3 (8.8%) had levels between 10% and 1%, and 5 (14.7%) had levels between 0.1% and 1%, whereas 3 (8.8%) had no baseline quantitative reverse transcription polymerase chain reaction available.
The median follow-up after starting asciminib was 14 months (range, 4-72). At the last follow-up, 26 patients (76.5%) were still using asciminib; 2 were on ponatinib, 1 was on dasatinib, 3 were undergoing chemotherapy, and 2 had undergone HSCT. The asciminib doses at the last follow-up were 20 mg once daily (1 patient), 40 mg once daily (2 patients), 60 mg once daily (1 patient), 40 mg twice daily (5 patients), 80 mg once daily (14 patients), 80 mg twice daily (1 patient), 160 mg twice daily (1 patient), and 200 mg twice daily (1 patient).
Response to asciminib
Among the 28 patients who initiated asciminib in CP, 2 (7%) had no response, whereas 26 (92%) achieved complete hematologic response (CHR). Thirteen patients (46%) achieved BCR::ABL1 transcripts <1% or complete cytogenetic response, 12 (42.8%) achieved MMR, and 4 (14.2%) achieved MR4.0/MR4.5. Among the 6 patients with AP or BC, 5 (83%) achieved CHR, 3 (50%) achieved complete cytogenetic response, and 1 had no response.
When responses were analyzed according to the indication for asciminib, 9 of 30 patients (30%) who switched due to resistance to a prior TKI achieved MMR or deeper responses, whereas 3 of 4 intolerant patients (75%) achieved MMR or deeper responses (P = .07). The MMR rate was 37.9% among patients without prior ponatinib exposure and 20% among those previously exposed to ponatinib.
Mutations were analyzed in 10 patients after asciminib resistance, among whom 4 (40%) had mutations (T315I, F359V, A337T, and T277A).
At the cutoff date, 26 patients (76.5%) remained on asciminib, whereas 8 patients (23.5%) discontinued treatment due to lack of efficacy. Six patients (17.6%) died, including 4 due to BC and 2 due to sepsis after disease progression (including 1 after HSCT; n = 2). Although the median OS was not reached, the mean OS was 45 months (±9 months), and the OS rate at 32 months was 48% (95% confidence interval, 28-68). The median follow-up among survivors was 16 months (range, 9-72).
Patients with T315I mutation
Among the 7 patients with the T315I mutation, 1 was in BC and 2 were in AP at asciminib initiation. Only 1 patient entered the MAP due to intolerance, whereas all others were resistant to prior treatment. All patients had previously received imatinib, and 5 had received dasatinib, 3 had received nilotinib, 2 had received ponatinib, and 1 had received asciminib 80 mg twice daily in a clinical trial. The last patient was discontinued from the trial due to resistance associated with the T315I mutation. All T315I mutation patients who entered the MAP initiated asciminib at 200 mg twice daily.
In terms of response, 5 patients (71%) achieved CHR, 1 achieved MR2, and 1 achieved MMR, whereas 2 patients had no hematologic response. One patient received ponatinib after asciminib failure and achieved a hematologic response; however, the patient died 1 year later from disease progression. Two patients also died due to progression to BC.
In terms of toxicity, 6 of 7 patients (86%) with the T315I mutation developed grade 3 or 4 hematologic toxicity (thrombocytopenia or neutropenia). Nonhematologic adverse events included grade 2 hyperbilirubinemia in 1 patient, grade 2 nausea and vomiting in another patient, and grade 2 generalized edema in a patient with concomitant metastatic melanoma.
Adverse events
In the total cohort, 16 patients (47.1%) presented with hematologic adverse events, including thrombocytopenia (26%) or neutropenia, grade 3 or 4 (14%), and 13 (38.2%) nonhematologic events (Table 3).
Hematologic and nonhematologic adverse events in 34 patients with CML treated with asciminib
| Event . | Grade 1-2, n (%) . | Grade ≥3, n (%) . |
|---|---|---|
| No. of patients with ≥1 adverse event (n = 10) | ||
| Hematologic toxicities | ||
| Thrombocytopenia | 09 (26.4) | 06 (17.6) |
| Neutropenia | 5 (14.7) | 5 (14.7) |
| Anemia | 0 | 2 (5.8) |
| Nonhematologic toxicities | ||
| Gastrointestinal | ||
| Diarrhea | 1 (2.9) | 0 |
| Constipation | 2 (5.8) | 0 |
| Nausea | 2 (5.8) | 0 |
| Cardiovascular | ||
| Acute myocardial infarction | 0 | 1 (2.9) |
| Hypertension | 0 | 1 (2.9) |
| Others | ||
| Anxiety | 1 (2.9) | 0 |
| Muscular pain | 2 (5.8) | 0 |
| Skin (rash, dryness, or pruritus) | 5 (14.7) | 0 |
| Upper airway infection | 2 (5.8) | 0 |
| Headache | 1 (2.9) | 0 |
| Edema | 1 (2.9) | 0 |
| Laboratory abnormalities | ||
| Increased serum amylase | 1 (2.9) | 0 |
| Increased blood bilirubin | 1 (2.9) | 0 |
| Increased aspartate aminotransferase | 1 |
| Event . | Grade 1-2, n (%) . | Grade ≥3, n (%) . |
|---|---|---|
| No. of patients with ≥1 adverse event (n = 10) | ||
| Hematologic toxicities | ||
| Thrombocytopenia | 09 (26.4) | 06 (17.6) |
| Neutropenia | 5 (14.7) | 5 (14.7) |
| Anemia | 0 | 2 (5.8) |
| Nonhematologic toxicities | ||
| Gastrointestinal | ||
| Diarrhea | 1 (2.9) | 0 |
| Constipation | 2 (5.8) | 0 |
| Nausea | 2 (5.8) | 0 |
| Cardiovascular | ||
| Acute myocardial infarction | 0 | 1 (2.9) |
| Hypertension | 0 | 1 (2.9) |
| Others | ||
| Anxiety | 1 (2.9) | 0 |
| Muscular pain | 2 (5.8) | 0 |
| Skin (rash, dryness, or pruritus) | 5 (14.7) | 0 |
| Upper airway infection | 2 (5.8) | 0 |
| Headache | 1 (2.9) | 0 |
| Edema | 1 (2.9) | 0 |
| Laboratory abnormalities | ||
| Increased serum amylase | 1 (2.9) | 0 |
| Increased blood bilirubin | 1 (2.9) | 0 |
| Increased aspartate aminotransferase | 1 |
Among the 27 patients without the T315I mutation at baseline who were treated at doses of 80 mg once daily or 40 mg twice daily, 6 patients (22%) developed grade 3 or 4 hematologic toxicity and 4 (15%) required temporary dose interruption followed by dose reduction due to grade 3 or 4 hematologic toxicity. At the last follow-up, 17 patients remained on the initial dose, whereas the dose of 1 patient was reduced to 60 mg, 2 to 40 mg, and 1 to 20 mg. The patient with the reduced dose to 20 mg was particularly challenging to manage, experiencing recurrent episodes of grade 4 thrombocytopenia requiring platelet transfusions and showing no treatment response.
A grade 3 cardiovascular event occurred in a 70-year-old man who was a former smoker with no prior cardiovascular history or risk factors. He developed an acute myocardial infarction 7 months after starting asciminib. He had previously been treated with dasatinib and nilotinib. At the time of the event, his asciminib dose had already been reduced to 40 mg daily due to neutropenia, and his treatment is ongoing.
No severe adverse events led to permanent discontinuation of asciminib. All treatment discontinuations were attributed to resistance.
Discussion
In this Brazilian cohort, most patients (88%) had resistance to prior TKIs, and 35% achieved MMR or better. These outcomes align with other MAP reports,6,7,11,17,18 despite a relatively short follow-up.
Patients who switched to asciminib due to intolerance achieved higher molecular responses (75%) than those switching for resistance (30%), with this pattern also being reported in Australian cohorts.7 This finding underscores the importance of treatment sequencing, as the earlier use of asciminib in cases of intolerance appears to maximize benefit.
Prior ponatinib exposure reduced response rates, consistent with findings from Cortes et al5 and Luna et al.17 Luna et al reported MMR and MR4.5 rates of 65% and 19%, respectively, in patients without prior ponatinib, compared with rates of 32% and 11%, respectively, among previously exposed patients.17 Our results mirrored this trend, confirming that ponatinib pretreatment limits subsequent asciminib efficacy.
Thrombocytopenia (26%) was the most common adverse event. However, cardiovascular events were rare in our cohort. One patient developed an acute myocardial infarction. In general, the incidence of arterial events was low in patients from MAP (Table 3). Other MAPs reported similarly low rates: the Dutch access program documented 1 non–ST-elevation myocardial infarction 9 days after asciminib initiation and the Milojkovic cohort described a single cerebrovascular accident.8,19 In Breccia et al study, patients with significant comorbidities achieved good responses and tolerated treatment well.6 Russian and Israeli MAPs also confirmed high response rates with few severe adverse events.9,11,12 In the ASCEMBL trial, arterial adverse events occurred in 3.2% of patients treated with asciminib, with a median follow-up of 1.2 years,4 and in 5.1% at 2.3 years.3
Hematologic toxicity grade 3 to 4 occurred in 86% of T315I patients treated with 200 mg twice daily, compared with 15% in those receiving 80 mg/d or 40 mg twice daily. These results highlight the need for careful monitoring and dose considerations, particularly in patients with T315I mutations. However, 3 of the 7 patients with T315I mutation initiated asciminib in advanced phases.
In terms of asciminib efficacy in patients with T315I mutations, a phase 1 trial of 45 CP cases treated with 200 mg twice daily reported BCR::ABL1 ≤1% in 62.2% and MMR in 48.9%. In that study, 29 patients (60.4%) had prior ponatinib exposure, and 50% had ponatinib resistance. A retrospective analysis showed MMR rates of 52.1% (95% confidence interval, 27.3-80.02) with asciminib and 61.5% with ponatinib, with no significant difference.20 Our cohort, although small and heavily pretreated (2 in AP, 1 in BC, and 2 with prior ponatinib exposure), achieved inferior results, with only 1 patient (14%) reaching MMR. These findings illustrate how disease phase and prior therapy strongly influence real-world responses.
Despite its limitations, including small sample size and population heterogeneity, this study provides, to our knowledge, the first real-world evidence of asciminib use in Brazil. Although asciminib was approved in June 2023 for CML in CP after ≥2 TKIs, asciminib remains unavailable in the public health care system. Ponatinib has also been approved but lacks reimbursement, leaving both therapies inaccessible to public health care and thus limiting patient care.
Thrombocytopenia emerged as the most frequent adverse event, and arterial events remained uncommon, consistent with MAP data and ASCEMBL.3
Overall, the safety profile supports broader use of asciminib for patients with CML and resistance or intolerance to ≥2 TKIs, provided patients undergo close cardiovascular monitoring, especially those with prior exposure to nilotinib or ponatinib.
In conclusion, asciminib represents an effective and well-tolerated treatment option for later lines of CML therapy. Patients without prior ponatinib exposure benefit most, highlighting the value of earlier access to asciminib.
Acknowledgments
The authors thank all involved centers for their support, Novartis’ Managed Access Program for providing asciminib to the patients, and Eliana Miranda for the support in the statistical analysis.
Authorship
Contribution: K.P. designed the research, collected data, analyzed data, and wrote the manuscript; and all authors collected data, contributed substantially to data analysis and interpretation, drafted and reviewed the manuscript, and gave final approval of the version to be published.
Conflict-of-interest disclosure: K.P. serves on the advisory boards of Novartis, Pfizer, GlaxoSmithKline, and Servier; is a speaker for Novartis, Pfizer, and Pint-Pharma; and has received travel support from Novartis. F.S.S. is a speaker for Novartis. V.F. is a speaker for and serves on the advisory board of Novartis. The remaining authors declare no competing financial interests.
Correspondence: Katia Pagnano, Centro de Hematologia e Hemoterapia, Universidade Estadual de Campinas, Rua Carlos Chagas 480, Campinas, SP 13083-878, Brazil; email: kborgia@unicamp.br.
References
Author notes
The data supporting the findings of this study are available on request from the corresponding author, Katia Pagnano (kborgia@unicamp.br), subject to review.