TO THE EDITOR:
Acute promyelocytic leukemia (APL) represents a distinct variant of acute myeloid leukemia and is marked by the t(15;17) translocation, a high risk of bleeding, and remarkable sensitivity to anthracyclines, all-trans retinoic acid (ATRA), and arsenic trioxide (ATO).1-3 The ATRA-ATO regimen has significantly transformed treatment, thereby eliminating chemotherapy for low-risk cases, forming the backbone for the treatment of high-risk disease, and leading to one of the highest cure rates in acute myeloid leukemia.4 However, significant disparities in access to optimal care persist between high-income countries and low- and middle-income countries (LMIC).
In Nepal, oncology services are limited to a few urban centers that face challenges, such as workforce shortages, inconsistent medical supplies, and financial constraints. Despite these obstacles, the Clinical Hematology Unit at Civil Service Hospital has been providing leukemia care since 2011.
We analyzed 90 consecutive patients, irrespective of age, who were diagnosed with APL between March 2015 to June 2024. A diagnosis of APL was based on the characteristic morphology of leukemic cells in peripheral blood and/or bone marrow and was confirmed by the presence of the PML::RARA fusion gene or the corresponding chromosomal translocation using fluorescence in situ hybridization, cytogenetics, or reverse transcription polymerase chain reaction. ATRA was initiated within 1 hour of clinical suspicion based on the identification of abnormal promyelocytes on a peripheral blood smear by residents and fellows who were well trained to recognize suspected APL on smears. Minimal residual disease status was monitored using reverse transcription polymerase chain reaction every 3 months for the first 2 years of follow-up. The median duration of follow-up was 6.2 years. The following data were extracted: demographics, laboratory values (white blood cell count and platelet count), treatment (ATRA, ATO, chemotherapy), adverse events, and survival. Overall survival (OS) was assessed using the Kaplan-Meier method and was compared between groups using the log-rank test. All analyses were performed using R, version 4.3.2. This study was approved by the ethical clearance committee of the Civil Service Hospital of Nepal. Informed consent was obtained from all patients included in this study.
The median patient age was 32 years (range, 14-71), with 62% classified as having low-risk APL (Table 1). Patients with low-risk APL received ATO plus ATRA daily until complete remission (CR) or up to 60 days, followed by ATO for 5 days per week on a 4 weeks on/4 weeks off cycle (total 4 courses) and ATRA on a 2 weeks on/2 weeks off cycle (total 7 courses), as specified in the Intergroup APL0406 protocol.5 Patients with high-risk APL received ATRA and daunorubicin for cycle 1, followed by 8 weeks of ATO and 2 cycles of ATRA and daunorubicin, and then 2 years of ATRA maintenance as specified in the North American Leukemia Intergroup Study with the omission of cytarabine during induction.6 Three patients relapsed after induction and underwent allogeneic hematopoietic cell transplantation (HCT); these were the only relapses to date.
Baseline characteristics and adverse event profile of the study population
| . | High risk (n = 34), n (%) . | Low risk (n = 56), n (%) . | P value . |
|---|---|---|---|
| Age, y | .09 | ||
| Mean ± SD | 38.09 ± 15 | 32.8 ± 11.9 | |
| Median | 36.5 | 32 | |
| IQR | 24.25-50.75 | 22.75-42.0 | |
| Sex | .74 | ||
| Male | 19 (55.9) | 28 (50) | |
| Female | 15 (44.1) | 28 (50) | |
| WBC count, per μL | <.001 | ||
| Median | 49 × 10³/μL | 3.4 × 10³/μL | |
| IQR | 28.625 × 10³/μL-70.332 × 10³/μL | 2.15 × 10³/μL-5.525 × 10³/μL | |
| Platelet count, per μL | .39 | ||
| Median | 17 × 10³/μL | 23 × 10³/μL | |
| IQR | 10.5 × 10³/μL-28.75 × 10³/μL | 12 × 10³/μL-33.25 × 10³/μL | |
| Febrile neutropenia | 29 (85.3) | 44 (78.6) | .60 |
| Coagulopathy | 26 (76.5) | 36 (64.3) | .33 |
| Fungal infection | 7 (20.6) | 17 (30.4) | .44 |
| Differentiation syndrome | 10 (29.4) | 15 (26.8) | .97 |
| Gastric ulcer | 1 (2.9) | 3 (5.4) | .99 |
| Intracranial bleed | 8 (23.5) | 1 (1.8) | .004 |
| CNS disease | 1 (2.9) | 0 | <.001 |
| Fournier gangrene | 0 | 3 (5.4) | .46 |
| Dilated cardiomyopathy | 2 (5.9) | 1 (1.8) | .67 |
| CR | 26 (76.5) | 55 (98.2) | .003 |
| Mortality | 8 (23.5) | 1 (1.8) | .004 |
| Transplant | 1 (2.9) | 2 (3.6) | .9 |
| Follow-up duration | .36 | ||
| Median | 2 082 | 2 268 | |
| IQR | 475-3 045 | 1 644-2 726 |
| . | High risk (n = 34), n (%) . | Low risk (n = 56), n (%) . | P value . |
|---|---|---|---|
| Age, y | .09 | ||
| Mean ± SD | 38.09 ± 15 | 32.8 ± 11.9 | |
| Median | 36.5 | 32 | |
| IQR | 24.25-50.75 | 22.75-42.0 | |
| Sex | .74 | ||
| Male | 19 (55.9) | 28 (50) | |
| Female | 15 (44.1) | 28 (50) | |
| WBC count, per μL | <.001 | ||
| Median | 49 × 10³/μL | 3.4 × 10³/μL | |
| IQR | 28.625 × 10³/μL-70.332 × 10³/μL | 2.15 × 10³/μL-5.525 × 10³/μL | |
| Platelet count, per μL | .39 | ||
| Median | 17 × 10³/μL | 23 × 10³/μL | |
| IQR | 10.5 × 10³/μL-28.75 × 10³/μL | 12 × 10³/μL-33.25 × 10³/μL | |
| Febrile neutropenia | 29 (85.3) | 44 (78.6) | .60 |
| Coagulopathy | 26 (76.5) | 36 (64.3) | .33 |
| Fungal infection | 7 (20.6) | 17 (30.4) | .44 |
| Differentiation syndrome | 10 (29.4) | 15 (26.8) | .97 |
| Gastric ulcer | 1 (2.9) | 3 (5.4) | .99 |
| Intracranial bleed | 8 (23.5) | 1 (1.8) | .004 |
| CNS disease | 1 (2.9) | 0 | <.001 |
| Fournier gangrene | 0 | 3 (5.4) | .46 |
| Dilated cardiomyopathy | 2 (5.9) | 1 (1.8) | .67 |
| CR | 26 (76.5) | 55 (98.2) | .003 |
| Mortality | 8 (23.5) | 1 (1.8) | .004 |
| Transplant | 1 (2.9) | 2 (3.6) | .9 |
| Follow-up duration | .36 | ||
| Median | 2 082 | 2 268 | |
| IQR | 475-3 045 | 1 644-2 726 |
CNS, central nervous system; IQR, Interquartile range; SD, standard deviation; WBC, white blood cell.
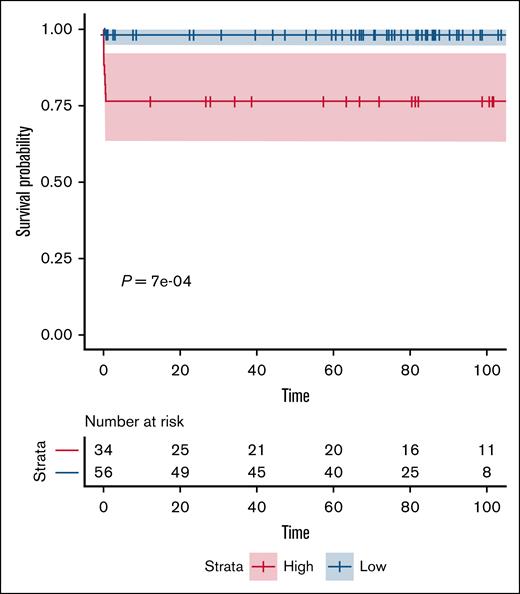
CR was achieved in 90% of patients (n = 81); 9 patients (10%) died during induction, all from intracranial hemorrhage (ICH). There were no other deaths during the follow-up. Three of these patients developed disseminated intravascular coagulation (DIC) and/or differentiation syndrome. Notably, 8 deaths occurred in the high-risk group. The 1- and 5-year OS for the entire cohort was 90% (95% confidence interval, 84.0-96.4) with all deaths occurring during induction and none thereafter. The outcomes were notably superior in the low-risk cohort when compared with the high-risk cohort, with the 1-year OS rates being 98.2% vs 76.5% (Figure 1). Except for the 9 patients who died during induction, all remaining patients were alive and in sustained remission at the date of publication, including the 3 patients who underwent allogeneic HCT. All patients remained minimal residual disease negative at 2 years.
Major adverse events included neutropenic fever (82%), coagulopathy (prolonged prothrombin time/international normalized ratio/activated partial thromboplastin time) (69%), differentiation syndrome (28%), pulmonary fungal infections (27%), acute heart failure (3.3%), foot gangrene (3.3%), and gastric ulceration (4.5%). All patients with coagulopathy had prolonged coagulation profiles and ecchymosis, and 3 patients met the International Society on Thrombosis and Haemostasias criteria (score ≥5) for overt DIC. All 3 patients with overt DIC developed ICH.
Despite the challenges of a resource-limited setting, we demonstrate a high response rate and favorable survival outcomes in our study. Our publicly funded tertiary hospital, Nepal’s largest hematology center that evaluates >150 new cases of acute leukemia per year and only HCT provider, serves patients from across Nepal and from bordering districts of India, making this cohort broadly representative of the Nepalese population with APL. Our CR rate (90%) exceeds those reported in Pakistan (86.3%) and Latin America (85.4%).7,8 The outcomes, especially in low-risk APL, are also consistent with the findings of Lo-Coco et al.5 The high CR rate in our cohort reflects reduced induction mortality, improved supportive care, rigorous clinician training in early APL recognition, and rapid ATRA initiation per protocol in the emergency department.
Historically, population-based studies have reported higher induction mortality (17%-29%), although they primarily included patients treated before 2000s when there was limited supportive care and an unclear proportion of patients with high-risk APL.9,10 The induction mortality observed in our high-risk cohort (24%) is similar to that of the North American Leukemia Intergroup Study that used ATRA and chemotherapy for induction.6 Consistent with previous studies, hemorrhage accounted for all deaths during induction in our cohort.11,12 In contrast, this mortality rate is still notably higher than the 5% to 15% reported in recent studies that employed ATO, chemotherapy, and gemtuzumab ozogamicin in varying combinations,13-16 although the differences in the treatment protocols with incorporation of ATO or gemtuzumab ozogamicin in the induction regimen may make it harder to compare the results directly. Our results show a slightly better induction mortality rate than the 28.5% early death rate reported among high-risk patients in India.17
The 1-year and 5-year OS rate of 90% is better than that reported in a study of 58 patients with APL from Pakistan and 238 patients from India.7,18 Our OS remains comparable with that reported in high-income countries, especially within the low-risk APL cohort.19 However, it is important to consider that the follow-up data are relatively immature at the time of publication. Six patients with low-risk disease had less than 1 year of follow-up with 4 of those patients being within 100 days of induction treatment.
Our study has a few limitations. Challenges in the diagnosis and access to care, especially for rural patients, may introduce selection bias. Our previous research, although not limited to APL, has shown that most individuals with acute leukemia in Nepal travel from outside Kathmandu (93%), are unemployed, and cannot afford private care, which drives severe financial toxicity.20 This underscores the urgent need for long-term government investment in cancer care infrastructure across the country. As a retrospective study, causality cannot be established, and unmeasured confounders (eg, treatment delays, nutrition, supportive care) may have influenced the outcomes. Quality of life metrics were also not assessed.
Despite the limitations, our study helps to prioritize policy interventions. A key challenge is ensuring reliable access to ATRA and ATO in LMICs. We are collaborating with the government of Nepal to include ATRA and ATO in the national essential drug list. Our use of generic ATO underscores a global disparity; many LMIC patients still lack access to this curative treatment because of the high costs.21 Broader policy recommendations also include enhancing laboratory capacity with advanced diagnostic techniques, increasing funding for cancer centers outside Kathmandu, and decentralizing the distribution of oncology professionals within the Ministry of Health and Population to strengthen cancer care services at the community level.
APL is highly curable with early diagnosis, prompt ATRA initiation, adherence to established treatment protocols, and focused clinical training. Generic ATO offers a cost-effective option for LMICs, which potentially improves global access to this life-saving treatment. ICH remains a major cause of early mortality, highlighting the need for enhanced supportive care during induction. These findings support the feasibility and effectiveness of delivering curative APL therapy in low-resource settings.
Contribution: B.S.P., R.S., B.P., T.I., S.G., and U.J. conceptualized the study; B.S.P., R.S., B.P., and T.I. performed the data collection; B.S.P. and U.J. conducted the literature review and drafted the manuscript; S.G. and U.J. were responsible for the statistical analyses; and all authors critically revised the manuscript, provided substantial feedback, and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bishesh Sharma Poudyal, Department of Clinical Hematology and Bone Marrow Transplantation Services, Civil Service Hospital, Minbhawan, New Baneshwor, Kathmandu 44600, Nepal; email: drbishesh78@hotmail.com.
References
Author notes
Original data are available on request from the corresponding author, Bishesh Sharma Poudyal (drbishesh78@hotmail.com).