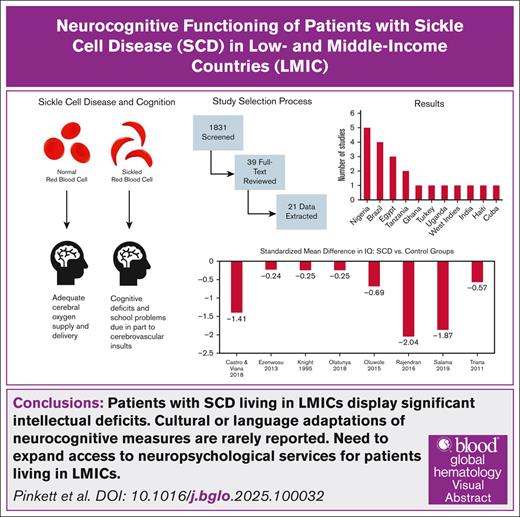
Visual Abstract
Sickle cell disease (SCD) is a monogenic blood disorder that affects the production of hemoglobin. Patients with SCD display slowed neurocognitive development resulting from a combination of cerebrovascular complications and sociodemographic factors. In low- and middle-income countries (LMICs), patients have limited access to neurocognitive surveillance. We sought to evaluate the neurocognitive impact of SCD in patients living in LMICs, establish which neurocognitive assessment measures are commonly used in this setting, and determine how these measures are contextually adapted. To answer these questions, we used an exhaustive literature search to systematically collect abstracts. Studies that focused on participants with SCD of any genotype and included a measure of cognitive/academic outcomes were included, whereas commentaries and editorials were excluded. A total of 1831 abstracts were screened, with 39 abstracts selected for full-text review and 21 articles used for data extraction. Most studies were conducted in Africa, Middle Eastern countries, and Brazil. Common measures included the Wechsler Intelligence Scale for Children or Adults. In 8 studies that included a measure of overall intelligence for patients with SCD and a control group, patients with SCD showed lower performance (z = −3.44, P = .0006) than controls. Information regarding cultural or language adaptations of neurocognitive measures was rarely reported, suggesting that neurocognitive measures are routinely used outside of the linguistic and cultural parameters for which they were developed. Further research is needed to validate the neurocognitive measures used in LMICs for patients with SCD.
Introduction
Sickle cell disease (SCD) is an inherited blood disorder that affects the production of hemoglobin and impairs the oxygen-carrying capacity of red blood cells.1 Although SCD is a monogenic disorder, extensive genetic variability (eg, hemoglobin genotype, haplotypes, and the number of α-globin genes) affects the disease phenotype. Environmental factors such as socioeconomic status also have a substantial impact on the disease. Over 90% of individuals with SCD living in high-income countries (HICs) survive to adulthood, whereas less than half living in low- and middle-income countries (LMICs) reach adulthood.2 These disparities are exacerbated by the concentration of over half of all SCD cases in 3 LMICs: Nigeria, Democratic Republic of Congo, and India.3 Approximately 500 000 people are born with SCD each year, and 8 million people are living with SCD globally.4 The number of people living with SCD is expected to increase in both HICs and LMICs due to a number of factors including advancements in SCD diagnostic tools, population growth, and global migration patterns, emphasizing the need to improve the quality of life for all patients with SCD.
Individuals with SCD experience damage to nearly every organ, including the brain.5 Common complications of SCD include episodes of vaso-occlusive pain, retinopathy, and pulmonary hypertension. Without treatment, stroke occurs in 7.4% and 11% of patients with the most severe forms of SCD by age 14 and 20 years, respectively.6 Silent cerebral infarcts (ie, focal brain lesions without acute behavioral change)7 occur in approximately half of these patients by adulthood.8 Both stroke and silent infarcts are associated with significant neurocognitive deterioration.9 Even in the absence of clear cerebrovascular complications, patients with SCD display neurocognitive deficits across most cognitive domains,9 likely resulting from chronic insufficiencies in oxygen delivery to the brain. Cognitive deficits in SCD are associated with functional consequences, including poor academic performance10-12; cognitive deficits and symptoms13; difficulties transferring to, and remaining in, adult health care14,15; limited employment opportunities16,17; and a poorer health-related quality of life.18-20 These negative adaptive outcomes exacerbate the significant economic burden of patients with SCD, and society.21,22
Numerous assessment tools are available to evaluate neurocognitive performance in patients with SCD in HICs. These neurocognitive assessments were developed, standardized, and validated among representative English-speaking populations and are designed to be used in similar contexts. They are used to evaluate strengths and weaknesses across cognitive domains, such as global intelligence, memory performance, executive functioning, processing speed, and motor functioning. These evaluations enable individuals with SCD to be referred for specialized interventions and petition to obtain support at school and work in some HICs.5
In LMICs, access to neurocognitive evaluations and associated services is limited.23,24 To support the development of these services, valid and reliable measures must be developed. Despite these gaps, numerous studies in LMICs are evaluating neurocognitive performance among patients with SCD. From a research perspective, the valid and reliable measurement of neurocognitive outcomes in LMICs is important to evaluate the influence of biomarkers, social determinants, and biomedical/behavioral treatments. Neurocognitive assessment tools may be used to screen for cerebrovascular risk25 and determine which patients need additional support during the transition to adult health care.26 Explaining the potential neuroprotective properties of disease-modifying treatments (eg, hydroxyurea)27 may further encourage adherence to such medications.
In addition to limited access to neurocognitive assessments and services, patients with SCD in LMICs have limited access to best practice neuropsychological services due to cultural and linguistic barriers.24 Despite considerable growth in the international neuropsychology community,24,28 most neurocognitive tests have been developed and validated in Western countries using Western English-speaking populations.29 Moreover, increased rates of diagnostic error are observed when a neurocognitive test developed in one culture is applied to another.30 Although there is some global variation in clinical neuropsychology training models, some core competencies are shared across models, including the need for competencies in diversity and culture with respect to clinical neuropsychological practice and use of validated and reliable measurement tools.31 Additionally, the International Neuropsychological Society’s Cultural Neuropsychology Special Interest Group has worked to extend the International Test Commission Guidelines for Translating and Adapting Tests32 to include specific recommendations for adapting/translating neuropsychological tests.33 They developed 18 guidelines that span the precondition, test development, confirmation, administration, score scales and interpretation, and documentation stages of the adaptation/translation of neuropsychological tests.33 Despite global agreement on cultural competencies in clinical neuropsychology and the availability of guidelines for the development of culturally and linguistically contextualized tests, it is unclear how frequently clinicians and researchers are using adapted or translated tests rather than unaltered neuropsychological instruments globally.
Reviews and meta-analyses have highlighted the cognitive and academic deficits that patients with SCD experience,9,34-38 but none of these reviews have focused on neurocognitive outcomes in LMICs. The most recent and comprehensive review of neurocognitive performance in SCD conducted by Prussien et al9,34 only included 4 studies performed in LMICs. Our objective was to quantitatively evaluate the neurocognitive outcomes of patients with SCD living in LMICs compared with those of normative expectations and controls in LMIC. Secondarily, we sought to review the specific neurocognitive assessment tools used in these studies and what, if any, adaptations were made to these tools for their use in LMICs.
Methods
Eligibility criteria
The inclusion criteria for study selection included the following: (1) the study included patients with any genotype of SCD; (2) a standardized measure of cognitive or academic outcomes was used; and (3) patients were from LMICs, defined in accordance with the World Bank country classifications in 2023. Studies were excluded for the following reasons: (1) patients had only sickle cell trait; (2) studies were conducted in upper-middle-income countries or HICs; or (3) studies were Comments or Editorials.
Information sources and search strategy
A search was performed using various search terms in the following electronic bibliographic databases: Embase, PubMed, Web of Science, Global Health CABI, Education Resources Information Center, Cumulated Index in Nursing and Allied Health Literature, and PsycINFO. MEDLINE was used by a medical librarian (J.W.) as the primary engine with the following concepts: (1) sickle cell disease, (2) academic or cognitive approaches, and (3) low- and low-middle-income countries. Medical Subject Headings terminology was identified, which enabled the grouping of keywords with various synonyms. Keywords were searched in the Ovid MEDLINE database using the title, abstract, and other keyword fields before translation into other databases. The initial search terms and strategy are provided in supplemental Table 1.
Study selection
The initial search identified 1831 abstracts. The abstracts were uploaded into Covidence, a software used to systematically perform title, abstract, and/or full-text screening of articles.39 All 1831 titles and abstracts were independently screened by 2 reviewers, and those meeting the inclusion and exclusion criteria were accepted. Any discrepancies were resolved within Covidence. Of 1831 abstracts screened, 39 studies met the initial criteria for full-text review. After the full-text review, 18 additional studies were excluded, resulting in a total of 21 studies (Figure 1).
Study selection flowchart showing the identification, screening, inclusion, and exclusion processes. CINAHL, Cumulated Index in Nursing and Allied Health Literature; ERIC, Education Resources Information Center.
Study selection flowchart showing the identification, screening, inclusion, and exclusion processes. CINAHL, Cumulated Index in Nursing and Allied Health Literature; ERIC, Education Resources Information Center.
Data extraction
Data were independently extracted by 2 reviewers. Study data included country, socioeconomic status, sample size, age range, sex, ethnicity, silent cerebral infarct status, SCD genotype, measures used, and performance on neurocognitive/academic outcomes.
Neurocognitive measures observed in the studies included domains such as intelligence quotient (IQ), working memory, processing speed, verbal functioning, and visual functioning. A list of measures from each domain is provided in supplemental Table 2. Because few studies measured specific neurocognitive domains, quantitative analyses could not be performed. All neurocognitive domain outcomes were converted to standard scores.
Of 21 studies from which data were extracted, only 12 included information on IQ scores and were quantitatively analyzed. These studies rarely reported whether a licensed psychologist supervised evaluations but used various measures such as the Wechsler Intelligence Scale for Children (WISC), Wechsler Adult Intelligence Scale (WAIS), Draw-A-Person Test (DAPT), Wechsler Preschool and Primary Scale of Intelligence (WPPSI), the Malin Intelligence Scale for Indian Children (MISIC), and Stanford-Binet Intelligence Scales; supplemental Table 2 provides additional details.
The quality of each study included in our analysis was independently assessed by 2 reviewers. A version of the National Institutes of Health Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies adapted by Prussien et al was used.40,41 Studies received 1 point for each criterion met, for a total score of 0 to 6, with a higher value indicating a higher study quality. First, a determination was made on whether the research question or objective was clearly stated. Second, the studies were evaluated on their sample characteristics, including whether the study population was clearly defined, whether the participation rate of eligible persons was at least 50%, and whether there was consistent recruitment and application of inclusion and exclusion criteria. Finally, it was determined whether a sample size justification or other power analysis was included and whether outcome variables were clearly outlined and defined. Discrepancies between scoring were resolved by the 2 reviewers and adjusted.
The sample sizes, means, and variation (eg, standard deviation [SD], confidence interval [CI] size) were obtained for controls and SCD populations for each study. In Nunes et al,42 the SD was not reported, we therefore estimated it using the scores reported for each participant. Castro and Viana43 (patients with SCD, n = 63; controls, n = 63) reported means and 95% CIs, from which we also estimated the SDs.43
Statistical analyses
We conducted a meta-analysis to combine findings from smaller studies to yield more precise estimates and to better understand the variability in contributing studies.42,43
Specifically, random effects meta-analysis models were used to synthesize estimates of the mean IQs of patients with SCD and control patients, the standardized mean IQ difference between patients with SCD and control patients, and the mean IQ difference between SCD studies and normative populations with a mean IQ of 100 and a SD of 15. Heterogeneity in effect sizes was assessed using the Cochran Q test and the I2 statistic.44 The I2 statistic is a measure of the inconsistency of studies in a meta-analysis and quantifies the variability attributable to the heterogeneity of studies. Diagnostic tests based on studentized residuals and Cook’s distances were used to identify possible outliers and influential studies.45 We used the rank correlation test46 and the regression test47 to assess funnel plot asymmetry. Please note that these diagnostic test results should be viewed with caution given their reliability with meta-analyses containing <10 studies. The meta-analysis was conducted in R (version 4.3.2)48 with the comprehensive metafor package (version 4.6.0).49
Results
Table 1 summarizes the study characteristics, including the country in which the study was completed; the sample size of the SCD group and control group (if included); the genotype(s) of the SCD group; the study quality; the neurocognitive and academic measures used; a description of test adaptations, if any were shared; and the primary study findings. Supplemental Table 3 details additional information for each study (if reported), including urban vs rural setting, and predictors of neurocognitive outcomes.
The 21 studies were conducted in 11 countries: Nigeria (5 studies), Brazil (4 studies), Egypt (3 studies), Tanzania (2 studies), Ghana, Turkey, Uganda, West Indies, India, Haiti, and Cuba (1 study each). The most frequently used measures across studies were the Weschler series of intelligence scales (ie, the WAIS fourth edition, WAIS revised, WISC third edition, WISC fourth edition, WISC revised, and WPPSI). Of the 12 studies that used a Wechsler intelligence scale or an adapted version of one (eg, the MISIC), only 2 studies51,64 administered select subtests instead of administering the full measure. Additionally, the Rey-Osterrieth complex figure test, Raven’s standard progressive matrices, Montreal Cognitive Assessment (MoCA), Tower of London, DAPT, and the Stanford Binet intelligence scales were used across >1 of the studies from which data were extracted. Three studies53,55,68 used only screener measures (eg, MoCA and DAPT), whereas the remaining 18 studies used comprehensive measures. Of the studies reviewed, 14 included a control group in their design: 5 studies used community controls, 2 used a sex-/age-matched control group, 2 studies used a healthy sibling control group, and 5 used an unidentified type of control group.
Of the 21 studies from which data were extracted, 6 indicated some level of adaptation/translation for the neuropsychological tests used. Of these 6 studies, 2 studies70,73 reported translating measures to the local language. The remaining 4 studies67,71,75,76 used versions of neuropsychological tests that had been adapted and standardized for the local culture/language. Rajendran et al71 used an adapted version of the WISC, whereas the remaining measures were translated into the local language. Additionally, although Montanaro et al67 used the Italian standardized versions of the WISC third edition and WPPSI, the study visits were conducted in either English or French. These data indicate that less than a third of the studies reviewed (28.5%) reported using culturally and/or linguistically adapted measures. Moreover, only 3 studies (14.3%) reported that all their measures were both culturally and linguistically adapted for the study context and that the measures were delivered in the local language. Only 7 of the reviewed studies reported the psychometric properties of their measures when used with non-Western populations.
Correlates of cognitive performance across domains are described in supplemental Table 3. Patient or parent education level was consistently associated with higher cognitive scores. Older age and the presence of cerebrovascular insults (eg, stroke or silent infarct) were associated with worse performance. Inconsistent findings were observed for laboratory values (eg, hemoglobin and oxygen saturation) and SCD genotype.
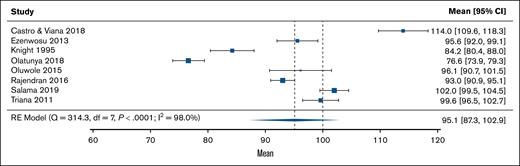
Of 12 studies that reported IQ, 8 included a control group. Figure 2 shows the IQ scores for these control groups. On average, the control group IQ was 95.1 (95% CI, 87.3-102.9), which was slightly below the normative expectations but not significantly different (z = −1.23; P = .22). The heterogeneity of effect sizes in controls was substantial (I2 = 98.0%; P < .0001). A funnel plot of these scores is shown in supplemental Figure 1 and no signs of asymmetry was found in the results (P = .90 and P = .50, respectively). For individuals with SCD (k = 12), the estimated IQ was 83.8 (95% CI, 79.9-87.7), which was significantly lower than normative expectations (z = −8.18; P < .0001; Figure 3). The heterogeneity of effect sizes in SCD samples was considerable (I2 = 92.7%; P < .0001). A funnel plot of these scores is shown in supplemental Figure 2 and no signs of asymmetry was found in the results (P = .31 and P = .89, respectively).
The mean control group IQ outcomes compared with normative expectations in a random-effects meta-analysis model. df, degrees of freedom; RE, random effects.
The mean control group IQ outcomes compared with normative expectations in a random-effects meta-analysis model. df, degrees of freedom; RE, random effects.
The estimated mean IQ of sickle cell disease groups compared with normative expectations in a random-effects meta-analysis model. df, degrees of freedom; RE, random effects; Std., standardized.
The estimated mean IQ of sickle cell disease groups compared with normative expectations in a random-effects meta-analysis model. df, degrees of freedom; RE, random effects; Std., standardized.
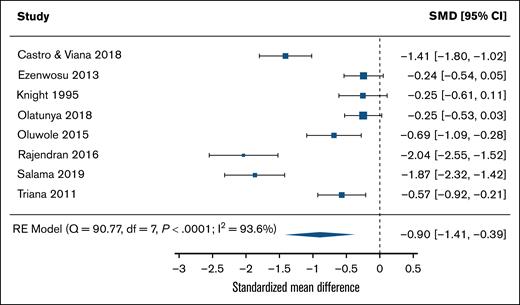
Figure 4 shows a comparison of the standardized mean difference in IQ scores between SCD and control groups. The estimated standardized mean difference based on the random effects model was −0.897 (95% CI, −1.41 to −0.39), indicating significantly worse performance in the SCD samples compared with control samples (z = −3.44; P = .0006). The heterogeneity of effect sizes in standardized mean differences of SCD vs control samples was considerable (I2 = 93.6%; P < .0001). All estimates (SCD minus control) were consistently negative, but 3 of the sample 95% CIs included 055,68,77. A funnel plot of the estimates is shown in supplemental Figure 3. Both the rank correlation and the regression test indicated potential funnel plot asymmetry (P = .0055 and P < .0001, respectively).
Standardized mean differences of IQs between sickle cell disease groups and control groups in a random-effects meta-analysis model. df, degrees of freedom; RE, random effects; SMDs, standardized mean differences.
Standardized mean differences of IQs between sickle cell disease groups and control groups in a random-effects meta-analysis model. df, degrees of freedom; RE, random effects; SMDs, standardized mean differences.
We analyzed the study quality of all 21 studies. The average quality score was 3.8 out of 6 for all studies combined, and 3.7 for the subset of 12 studies that included IQ scores of patients with SCD. Most studies did not report a participation rate or a sample size justification.
Discussion
Patients with SCD are at a high risk for early and progressive neurocognitive deficits. Studies in HICs have highlighted these deficits and described the risk/resilience factors associated with neurocognitive performance. However, our understanding of neurocognition for patients living in LMICs is limited. Consistent with studies in HICs, we observed that patients with SCD display deficits in overall intellectual functioning when compared with normative expectations and matched controls. Several studies reported that lower socioeconomic status, older age, and the presence of cerebrovascular insults were associated with poorer cognitive scores. However, our ability to draw conclusions was limited by the substantial variability in the methodologies used to assess these outcomes. Most studies only reported global intelligence rather than more specific cognitive domains such as executive functioning, processing speed, or perceptual reasoning.
There was significant heterogeneity in the mean IQ scores across studies, with >90% of variability across studies being attributable to study heterogeneity. Our standardized mean difference analysis revealed several outlier studies, with significant deviations from 0 in a random effects model. Notably, the control groups in 2 of the studies77,68 performed more than an SD below the normative expectations. The poor performance of these control groups may be due, in part, to socioeconomic factors, or the psychometric properties of the measures used within that cultural context. Only 7 studies used fully adapted assessments, whereas 8 used assessments that were only translated, validated, or standardized to the population’s primary language. This is consistent with global reviews of neuropsychology services and research,23,24 which have shown that neuropsychology assessments are typically Western in origin, occasionally modified for international contexts, and rarely developed for use within a particular culture. Moreover, global neuropsychology services and studies have primarily included participants with high socioeconomic status, because financial and geographic constraints preclude many LMIC residents from accessing neuropsychological resources.
Worldwide, culturally adapted neurocognitive tests are sparse. Although most LMICs use primary languages other than English, few of the studies we evaluated had adapted assessments to their participants’ specific contexts. The goal of test adaptation is to enhance participant understanding by modifying questions without removing the concept being assessed, thus aligning the test with relevance to a cultural setting rather than simply transforming linguistics.78 Most studies used translation of the testing language to the population’s primary language to ensure surface-level understanding of the assessment, but most tests were not fully adapted. To our knowledge, full cultural adaptations of the WISC exist in France, Germany, and Sweden.79 Teams of local experts in language and intellectual assessment validate the changes made to each item by evaluating subtests such as similarities, information, comprehension, and vocabulary.80 Similarly, the MISIC has been adapted in Hindi and Marathi from the WISC.81 The revision involved all sections and subtests but primarily focused on the culturally biased verbal items.72 A neurocognitive screening tool for adults, the MoCA, has been adapted to several cultures whose languages include Sinhala, Turkish, and Portuguese, which are common languages of the studies we reviewed here.82 In these adapted versions, modifications were made to items and tasks that measure visuospatial ability, attention, language, memory, abstraction, and orientation. Semantics and linguistics were often changed to incorporate popular words, objects, animals, and other cultural items.83
Most neurocognitive assessment tools adhere to the Western culture and were normed on individuals who speak English. Use of these tools is predicated on the foundation that data generated must be reliable and valid. The application of Western tools in other cultural and linguistic contexts raises concerns regarding the reliability and validity of the data. The Culture-Language Interpretive Matrix was developed to assess the validity of a measure for culturally and linguistically diverse individuals from a normative group.84 By measuring the degree of cultural and linguistic demand, the Culture-Language Interpretive Matrix can hypothesize the validity of test scores by assigning participants certain profiles. This tool could be helpful in validating new, culturally adaptive measures by distinguishing target populations from other cultures.84 Although there is no clear criterion to adapt all neurocognitive assessments, some guidelines have recently been proposed, including the 18-item checklist outlined by The International Test Commission.85 Specifically, Khan et al described the steps taken to create guidelines for the adaptation of the MoCA, a measure commonly adapted for LMICs.86 Proceeding item by item, this group culturally and linguistically adapted each question, starting with translation and alphabet modification. By redesigning each question or image for a specific culture, the investigators consider the demographics of the intended users to find cultural familiarity behind the meaning of words. For example, in Indonesia, “lion” was replaced with “elephant” to enhance local understanding in cognitively healthy individuals.86 The development of more culturally and linguistically adapted tests could improve SCD health care globally.
This study highlights the potential limitations of implementing neuropsychological services in LMICs. The development and adaptation of culturally appropriate neuropsychological tools for LMICs is urgently needed. Therefore, future research should aim to adapt measures from non-Western populations by modifying specific test items, stimuli, and other culturally biased elements of testing. Additionally, open-source test development would enable the expansion of access in lower-resourced regions. More broadly, efforts are needed to train local providers and disseminate neuropsychological services. Collaborations with local stakeholders should involve the use of implementation and dissemination science tools to evaluate how neuropsychological services can be applied in LMICs. The maintenance of such services can be strengthened through the development of local neuropsychological training programs. Indeed, Ponsford reviewed global neuropsychological training efforts and services and found that most neuropsychologists working in LMICs were trained in Western countries, and many others did not return to their home countries after receiving training abroad.24 Additionally, most LMICs do not have dedicated neuropsychology training programs or certifications.24 However, several LMICs represented across the studies reviewed here do have hospital institutes or universities that offer dedicated neuropsychology training, including Brazil, India, and South Africa.24 Moreover, Master’s level neuropsychology programs are more common in LMICs such as Zambia and Guatemala.24 Given the variability in training, certification, and availability of providers in LMICs, global efforts to improve neuropsychological training and access to care will depend on the improvement of funding for dedicated neuropsychology positions, the use of a range of providers to meet the need within these countries, and appropriate reimbursement for their services. Ideally, individuals qualified to administer neurocognitive testing in the local language would be recruited from hospitals or other local organizations. An introduction to testing materials/manuals and administration strategies, such as question rehearsal and efficiency, should be given by supervisors. Evaluators should be expected to practice test administration and participate in fidelity assessments under supervisor observation to ensure successful training and accurate implementation of the services.87
This systematic review and meta-analysis were limited by the small number of eligible studies and inconsistent/insufficient data reporting. In addition, we were unable to examine group differences in neurocognitive domains beyond overall intelligence. Furthermore, inconsistent reporting precluded the investigation of correlates (eg, age and infarct status) of neurocognitive performance. Few studies recorded whether their participants were recruited in urban or rural areas. Due to population size and availability, participants were most likely selected from urban academic centers, which potentially reduces the generalizability of our findings to more rural populations. As this literature expands, more definitive conclusions can be drawn.
In conclusion, our review highlights that patients with SCD living in LMICs display significant deficits in measures of global intellectual functioning. Several studies documented difficulties in other neurocognitive domains (eg, processing speed and executive functioning); however, gaps and inconsistencies in the measurement of neurocognitive performance limited conclusions regarding deficits of specific domains in LMIC populations. Factors contributing to cognitive deficits appear consistent with those of HICs, including low socioeconomic status, older age, and cerebrovascular insults. Our systematic review identified that very few studies had adapted assessments to their participants’ specific contexts (eg, local culture and vocabulary), raising concerns regarding the reliability and validity of measurement. There is a critical need to expand access to neuropsychological services for patients with SCD in LMICs, including greater test adaptation and training of providers on how to use such adapted tools. Formal test adaptation should follow the guidelines proposed by the International Test Commission.85 Furthermore, the identification of neurocognitive deficits should be paired with evidence-based treatments known to be neuroprotective, including widespread stroke screening with transcranial Doppler ultrasound, expansion of hydroxyurea use, and access to chronic blood transfusions for stroke prevention. These measures, used in consonance with greater identification of neurocognitive deficits, are likely to limit the observed deficits for some patients living in LMICs.88 Finally, open-access dissemination of training materials and engagement of governments in the implementation of policies that ensure funding and access to neuroprotective therapies,89,90 will help promote optimal neurocognitive outcomes for children with SCD in LMICs.
Acknowledgment
A.M.H. was supported by National Heart, Lung, and Blood Institute (K23HL166697) during the time of this study.
Authorship
Contribution: A.M.H., J.N.L., and J.S.H. conceptualized the research question and design; J.W. created the search terms, performed the systematic literature search, and identified studies for inclusion; B.P. and K.N. independently extracted data from the selected studies; J.G. and G.K. conducted statistical analysis; B.P. wrote the initial draft; K.N. and E.R. contributed to manuscript revisions; and A.M.H. supervised the project and reviewed the final version of the manuscript.
Conflict-of-interest disclosure: J.S.H. receives royalties from UpToDate. The remaining authors declare no competing financial interests.
Correspondence: Andrew M. Heitzer, Department of Psychology and Biobehavioral Sciences, St. Jude Children’s Research Hospital, 262 Danny Thomas Pl, Memphis, TN 38105; email: andrew.heitzer@stjude.org.
References
Author notes
Raw data from the project are available on reasonable request from the corresponding author, Andrew M. Heitzer (andrew.heitzer@stjude.org).
The full-text version of this article contains a data supplement.