Key Points
We present, to our knowledge, the first Latin America description of this recently described disorder, considered an emergent disease.
It is critical to improve the investigation and follow-up of patients with germ line predisposition in low- and middle-income countries.
Germ line predisposition to myeloid neoplasms due to ERCC6L2 variants has been recently described as an emerging disorder. We present a 34-year-old female Brazilian patient who was diagnosed with myelodysplastic syndrome. During investigation, a novel homozygous missense variant in exon 10 of Excision repair cross-complementing 6 like 2 (ERCC6L2), NM_020207.7 (ERCC6L2:c.1583T>A; p.Leu528His), was found. A somatic next-generation sequencing panel showed TP53 p.R248W (variant allele frequency, 60%). The patient was successfully treated with a fully matched myeloablative allogeneic hematopoietic stem cell transplant.
Introduction
Excision repair cross-complementing 6 like 2 (ERCC6L2) is one of the most recently described genes associated with congenital bone marrow (BM) failure, with the first description in 2014.1 Germ line predisposition due to ERCC6L2 variants is now considered by World Health Organization 2022 classification2 as an emerging disorder.
Mutations in ERCC6L2 are associated with BM failure syndrome 2 (OMIM 615667),3 an autosomal recessive disorder with a high penetrance, characterized by BM failure, erythroid predominance, myeloid malignancies, somatic TP53-mutated clones, and, sometimes, learning disabilities and microcephaly.1 The protein encoded by this gene participates in nucleotide excision repair and nonhomologous end joining, mitigating replication stress and promoting centromere stability. In addition, it plays a role in mitochondrial function.1
Recently, Hakkarainen et al4 published extensive data of 52 patients from 35 families across 11 global centers with ERCC6L2 biallelic germ line variants. Patients presented more commonly with BM failure and hematologic malignancy, with a young average age of onset (37 years). More reports have been published in the last few months, for example, the first description of 2 cases in Spain, with no extrahematopoietic symptoms.5
Case description
We present a 34-year-old female patient without prior comorbidities who underwent investigation due to a 6-month history of asthenia. The patient has 2 siblings: 1 had transient childhood thrombocytopenia, successfully treated with corticosteroids, whereas the other had no relevant medical history. Familial history revealed no consanguinity, with 1 cousin having died from leukemia at an unknown age (Figure 1A).
Clinical presentation of the patient. (A) Genogram for the ERCC6L2-mutated pedigree. The squares denote males, and the circles denote females. The pink arrow shows the proband. The red boxes indicate individuals with MDS/AML. ERCC6L2 mutations were detected in the MDS/AML sample for III-3, but DNA was not available for the other affected individual (III-6). The gray box indicates individuals with detectable ERCC6L2 homozygous mutation without myeloid malignancy (III-1). The hatched box indicates an heterozygous carrier (III-5). No DNA was available from other family members. (B-D) Aspirate showing binuclear erythroblast (A), hyposegmentation of granulocytes (red arrow), (B) and monolobated megakaryocyte; (C) Leishman stained (original magnification ×400 to ×1000). (E) Trephine biopsy showing a hypercellular BM; hematoxylin and eosin–stained section (original magnification ×200). (F) Metaphase representative of the monosomal and complex karyotype 43,XX,der(5)t(5;17)(q14;q21),-7,dic(9;?)(p13;?),-17.- 18[19]/46,XX[1].
Clinical presentation of the patient. (A) Genogram for the ERCC6L2-mutated pedigree. The squares denote males, and the circles denote females. The pink arrow shows the proband. The red boxes indicate individuals with MDS/AML. ERCC6L2 mutations were detected in the MDS/AML sample for III-3, but DNA was not available for the other affected individual (III-6). The gray box indicates individuals with detectable ERCC6L2 homozygous mutation without myeloid malignancy (III-1). The hatched box indicates an heterozygous carrier (III-5). No DNA was available from other family members. (B-D) Aspirate showing binuclear erythroblast (A), hyposegmentation of granulocytes (red arrow), (B) and monolobated megakaryocyte; (C) Leishman stained (original magnification ×400 to ×1000). (E) Trephine biopsy showing a hypercellular BM; hematoxylin and eosin–stained section (original magnification ×200). (F) Metaphase representative of the monosomal and complex karyotype 43,XX,der(5)t(5;17)(q14;q21),-7,dic(9;?)(p13;?),-17.- 18[19]/46,XX[1].
Initial blood tests revealed anemia (hemoglobin, 6.3 g/dL), neutrophil count of 0.89 × 109/L, and platelet count of 55 × 109/L. The patient did not have any nonhematologic manifestations. BM aspirate revealed dysplasia of the 3 cell lines, with binuclear erythroblasts, hyposegmentation of granulocytes, and monolobated megakaryocytes (Figure 1B-D), with 2% blasts. BM biopsy showed a hypercellular marrow (100%) with 2% CD34+ cells (Figure 1E). Additionally, cytogenetic analysis revealed a monosomal and complex karyotype, including deletion 17p (Figure 1F). A diagnosis of myelodysplastic syndrome (MDS) was established.
To complement the investigation, whole-genome sequencing of a peripheral blood sample was performed through The Brazilian Rare Genomes Project.6 Data analysis through the Varstation platform revealed a homozygous missense variant in exon 10 of ERCC6L2, NM_020207.7 (ERCC6L2:c.1583T>A (p.Leu528His). This variant is within a 36.7-Mb segment of region of homozygosity in chromosome 9, also seen on other chromosomes, without evidence of copy number variations, which indicates parental consanguinity. The c.1583T>A variant is extremely rare, present in heterozygous state in only 2 of 492 479 individuals at the gnomAD Browser v.4.0.0 database7 and found once (the present patient) within 4390 individuals from our internal database. We also searched the Online Archive of Brazilian Mutations (ABraOM), without reports.8 Analysis by REVEL9 showed a score of 0.81, which predicts a damaging effect on the protein. Furthermore, the patient harbored a mutation in exon 7 of TP53, NM_000546.6(TP53):c.742C>T (p.Arg248Trp), with an allele frequency of 17%, suggestive of a somatic event. This TP53 variant has been widely described as pathogenic for both somatic and germ line occurrences.10
To date, literature reports only 1 patient described by Hakkarainen et al4 with a deleterious variant in exon 10 of ERCC6L2, a homozygous frameshift variant, c.1606_1751del (p.Leu536GlyfsTer18). This patient also presented with a somatic variant in TP53, c.745A>T (p.Arg249Trp), closely related to the somatic variant (p.Arg248Trp) found in our patient.
After the proband investigation, both siblings were genotyped for the c.1583T>A (p.Leu528His) variant in ERCC6L2. Results showed that one sibling (III-2) was heterozygous for ERCC6L2 mutation, whereas the other (III-5), with a history of transient childhood thrombocytopenia (now with platelet count 129 × 109/L), was homozygous for the variant (Figure 1A).
After 3 months (while she was waiting for whole-genome sequencing results), the patient showed an excess of blasts (12.4% aspirate and 15%-20% CD34+ in biopsy), with a myeloid phenotype (CD7par/CD13+++/CD33+/CD34/CD45++/CD71+/CD117/HLA-DR). A somatic next-generation sequencing panel (Ion Torrent, Oncomine Myeloid Assay Panel) showed TP53 p.R248W (variant allele frequency [VAF], 60%) and CEBPA p.H59Afs∗84 (VAF, 34.2%). It is important to highlight the increase of TP53 VAF, probably due to the increase in BM blasts. Specifically in ERCC6L2 disease, a study has shown that hematopoietic stem cell progenitor defect caused by ERCC6L2 mutations is completely rescued upon acquisition of additional TP53 mutations, albeit at the expense of an increased potential for malignant transformation.11
Due to this evolution, the patient was started on azacitidine treatment. After 2 cycles, a decrease in blast count was seen, although little improvement was observed in cytogenetics (Figure 2). We decided to proceed with a fully matched myeloablative (busulfan/fludarabine) allogeneic hematopoietic stem cell transplant (HSCT), using the HLA-identical brother (III-5) as donor. Because disease manifestation occurs exclusively in individuals with biallelic pathogenic variants in ERCC6L2 and no alternative donors were available for the patient, the heterozygous carrier brother (III-5) was selected as the donor. Platelet and neutrophil recovery occurred on days 12 and 16 after transplant, respectively. BM evaluation on day +30 showed a global hypocellularity, negative measurable residual disease (MRD) by flow cytometry and complete chimerism on karyotype and short tandem repeats. On day +60, there was evidence of positive MRD (0.67% of abnormal immature myeloid cells in BM), and immunosuppressant withdrawal was started. During this process, MRD became negative; however, symptoms of cutaneous graft-versus-host disease developed. Graft-versus-host disease was successfully treated with steroids. One and a half years after HSCT, the patient is now aged 35 years and remains MRD negative, with normal blood counts.
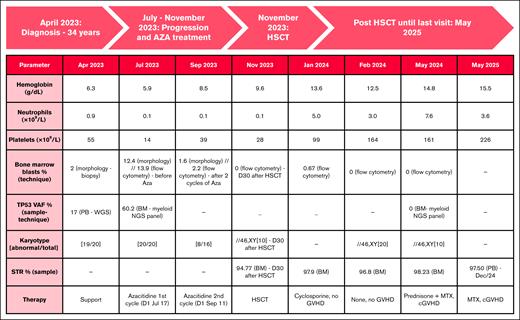
Longitudinal clinical, laboratory, cytogenetic, and therapeutic data. Trilineage hematologic recovery and full donor chimerism were achieved, with molecular response (TP53 VAF clearance), sustained remission, and complete chimerism. Two cycles of azacitidine were applied according to the service protocol, and the dose of azacitidine was 75 mg/m2 per day for 7 days. For each cycle, the whole dose is calculated in milligrams and divided along the week to receive 1 or 2 complete vials per day.12 The second cycle was postponed due to serious infectious complications (cellulitis). AZA, azacitidine; cGVHD, chronic graft-versus-host disease; MTX, methotrexate; PB, peripheral blood; STR, short tandem.
Longitudinal clinical, laboratory, cytogenetic, and therapeutic data. Trilineage hematologic recovery and full donor chimerism were achieved, with molecular response (TP53 VAF clearance), sustained remission, and complete chimerism. Two cycles of azacitidine were applied according to the service protocol, and the dose of azacitidine was 75 mg/m2 per day for 7 days. For each cycle, the whole dose is calculated in milligrams and divided along the week to receive 1 or 2 complete vials per day.12 The second cycle was postponed due to serious infectious complications (cellulitis). AZA, azacitidine; cGVHD, chronic graft-versus-host disease; MTX, methotrexate; PB, peripheral blood; STR, short tandem.
The 34-year-old brother, who was homozygous for the ERCC6L2 variant, was investigated, and a BM evaluation was performed, showing hypocellularity (35% of hematopoietic cells) with <1% of myeloid immature cells and a normal immunophenotype. He had no cytopenias in his last evaluation. No pathogenic TP53 mutation was detected.
Results and discussion
Investigation of germ line predisposition in patients with hematologic neoplasms is traditionally performed using DNA extracted from nonaffected neoplastic tissue, because the BM and peripheral blood may be affected with somatic variants, making it challenging to determine the origin of these variants.13 Although fibroblasts from skin biopsy culture are considered one of the reliable tissues, the process usually takes time (20-30 days from culture). Thus, we considered the high VAF (≥30% as a recommended threshold) associated with positive family history, as well as the same variant found in her siblings, as sufficient proof of germ line origin. It is important to highlight that this strategy was useful for ERCC6L2 because it is not a target of somatic mutation in MDS or acute myeloid leukemia (AML). Besides, another proof of germ line origin that could be used is the persistence of the VAF in remission or in DNA from tissue not contaminated by somatic mutations, such as cultured skin fibroblasts.
Regarding treatment, evidence in literature is so far scarce. In patients with MDS evolution, especially with TP53 mutations, HSCT is recommended, despite the dismal prognosis.14 It is also known that performing allogeneic HSCT before hematologic malignancies ameliorates the prognosis. However, the timing of HSCT should be weighed together with transplant-related complexities and with individual circumstances. During follow-up, frequent surveillance with repetitive blood tests, including screening for TP53 mutation is recommended. For those with TP53 mutations, an annual BM analysis is also suggested.4
Few case reports present data of hypomethylating agents in MDS before HSCT. One cites stable disease before HSCT; another showed complete morphological remission with incomplete hematologic recovery.4 In AML cases, intensive chemotherapy has already been described. After HSCT, there is an ongoing study evaluating the feasibility of prophylaxis with decitabine combined with filgrastim for children and young adults with AML, MDS and related myeloid malignancies, including patients with ERCC6L2 mutations (www.ClinicalTrials.gov identifier: NCT05796570).15
In conclusion, we report, to our knowledge, the first description of MDS caused by a ERCC6L2 variant in Latin America. We highlight the importance of studying germ line predisposition in not only patients at a young age but also in patients with physical manifestations, family history, other primary malignancies, and VAF close to 50%.13 We believe that we should consider routine screening in all suspect patients, despite age, because this information could potentially change follow-up and treatment. However, routine screening, due to its rarity, is not universally recommended yet, and this gene is not included in many commercial clinical genetic sequencing panels for myeloid neoplasms and germ line diseases.
We also emphasize that this homozygous variant described has never been associated with BM failure syndrome 2, thus contributing to the small worldwide cohort. Besides, our case strengthens the co-occurrence of TP53 mutations and the importance of HSCT. Finally, we would like to raise an important difficulty, especially in low- and middle-income countries, which is managing the appropriate follow-up and early treatment of relatives, not only in ERCC6L2-associated disorder but in all germ line diseases.
The study was approved by the research ethics committee of the Hospital das Clínicas da Faculdade de Medicina da USP (CAAE: 57965622.7.0000.0068) in accordance with the guidelines of the Declaration of Helsinki.
Acknowledgments
The authors are grateful for the collaboration of the patient and her family; to Denise Pasqualin from Pathology Department of Hospital Israelita Albert Einstein; to all technicians of cytogenetic laboratories of Hospital Israelita Albert Einstein and Hospital das Clínicas da Faculdade de Medicina da USP; especially to Aline de Medeiros Leal and Mauren Fernanda Moller dos Santos; and to Brazilian Rare Genomes Project team in Hospital Isrealita Albert Einstein.
Whole-genome sequencing study was funded by Brazilian Rare Genomes Project, a public‒private partnership with Hospital Israelita Albert Einstein and Programa de Apoio ao Desenvolvimento Institucional do Sistema Único de Saúde (PROADI-SUS) from the Brazilian Ministry of Health (Law 12.101/2009; protocol number 25000.083098/2019–71).
Authorship
Contribution: J.R.M.C, V.W., L.N., J.B.O.F., and T.F.A. analyzed data; V.W., J.V.R.O., and E.D.R.P.V. wrote the manuscript; and C.R.S.P., T.D.M.P., V.G.R., and E.D.R.P.V. designed the research and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Viktoria Weihermann, Service of Hematology, Transfusion and Cell Therapy and Laboratory of Medical Investigation in Pathogenesis and Directed Therapy in Onco-Immunohematology (LIM-31), Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, Avenida Dr Enéas Carvalho de Aguiar, 255 São Paulo, Brazil 05403-000; email: vikweihermann@gmail.com.
References
Author notes
J.R.M.C. and V.W. contributed equally to this study.
Data are available upon request from the corresponding author, Viktoria Weihermann (vikweihermann@gmail.com).

![Clinical presentation of the patient. (A) Genogram for the ERCC6L2-mutated pedigree. The squares denote males, and the circles denote females. The pink arrow shows the proband. The red boxes indicate individuals with MDS/AML. ERCC6L2 mutations were detected in the MDS/AML sample for III-3, but DNA was not available for the other affected individual (III-6). The gray box indicates individuals with detectable ERCC6L2 homozygous mutation without myeloid malignancy (III-1). The hatched box indicates an heterozygous carrier (III-5). No DNA was available from other family members. (B-D) Aspirate showing binuclear erythroblast (A), hyposegmentation of granulocytes (red arrow), (B) and monolobated megakaryocyte; (C) Leishman stained (original magnification ×400 to ×1000). (E) Trephine biopsy showing a hypercellular BM; hematoxylin and eosin–stained section (original magnification ×200). (F) Metaphase representative of the monosomal and complex karyotype 43,XX,der(5)t(5;17)(q14;q21),-7,dic(9;?)(p13;?),-17.- 18[19]/46,XX[1].](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodglobal/1/3/10.1016_j.bglo.2025.100022/1/m_bglo_bgh-2025-000171-gr1.jpeg?Expires=1767931614&Signature=C5IpNw7R6u62sah0e02nDoCccB-tlZDgTCKoGoym-UeVZp17amHzNp8zeloyteWUYwEuxEqQ~r~UiWZd5cuAMouD8q4p-mJyX69MuJj129D4mW3Oq34LDGNvIYSyqO7gY8XXPdMrlkzzGyxUJc9MggcwWW3ycdHXqy1WD8uh~1bz-kzwRATgdmJsPcMKiO0~tqgYpKGpVUZkhFzkBBfvHF4PKhR8zJTBHCHQsQGqAc3L-5zybmAgd9k2wKrx3mtW1tIZP7--x5s9rlXKC99r7ZLuQ70Pw9VhUzzND2IchOK4tHS1cdz2ytlfm98u~WfW5zcJ4sW8MapjvHZ8OhoPqg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)