Key Points
Talquetamab had the lowest rate grade 3/4 infections and discontinuations and deaths due to infections among T-cell engaging antibodies.
Results support talquetamab as a B-cell sparing treatment to allow preservation of key elements of humoral immunity for patients with RRMM.
Visual Abstract
Talquetamab is the first approved GPRC5D-targeting bispecific antibody for the treatment of relapsed/refractory multiple myeloma (RRMM), based on results from the phase 1/2 MonumenTAL-1 study. We report the infection profile among patients treated with talquetamab in MonumenTAL-1. Patients with triple-class exposed RRMM received subcutaneous talquetamab 0.4 mg/kg weekly or 0.8 mg/kg every other week (EOW). Patients with prior T-cell redirection therapy (TCR) were included in a separate cohort and received either schedule. Infections (graded by Common Terminology Criteria for Adverse Events v4.03) were managed per local guidelines. Patients received talquetamab (N = 339) with a median follow-up of 18.8 (weekly; n = 143), 12.7 (EOW; n = 145), and 14.8 (prior TCR; n = 51) months. Infections occurred in 58.7%, 66.2%, and 72.5% of patients, respectively; most common were respiratory infections, including COVID-19. Grade 3/4 infections occurred in 21.7% (weekly), 15.9% (EOW), and 27.5% (prior TCR) of patients, onset most common in cycles 1/2. Opportunistic infections were low (3.5%, 5.5%, and 5.9%, respectively). Five patients died due to infections. Neutrophil levels recovered at cycle 2 and were maintained throughout treatment. B-cell levels remained stable in early cycles, with notable increases at cycle 7. Immunoglobulin G levels recovered after cycle 3 and increased through cycle 17. Few patients started IV immunoglobulin following talquetamab (9.8% [weekly], 6.9% [EOW], and 5.9% [prior TCR]). Patients treated with talquetamab demonstrated relatively low rates of grade 3/4 infections and preservation of humoral immunity, distinguishing talquetamab as an important and potentially less immunosuppressive, novel treatment option for patients with RRMM. These trials were registered at www.clinicaltrials.gov as #NCT03399799 and #NCT04634552.
Introduction
Patients with multiple myeloma (MM) are at increased risk of infection, and complications associated with infections remain the leading cause of death in myeloma.1 The increased risk of infection is caused by multifactorial immunodeficiency, including disease-related deficits in humoral and cellular immunity, and patient-related factors such as previous therapies.1 The cumulative effects of immunodeficiency leave the immune system unable to eradicate tumor cells or mount effective responses against pathogens, impacting strategies for myeloma treatment, infection management (eg, prophylaxis including growth factors, supplemental immunoglobulin, vaccines, and antivirals), risk of hospitalization, cost of care, and survival.2-5
Immunotherapies that target normal immune cell populations may impair immune function and contribute to increased risk of infection.3 Novel therapies, such as anti-CD38 monoclonal antibodies and B-cell maturation antigen (BCMA)-targeting drugs, have improved efficacy outcomes for patients with MM. However, these agents may lead to an increased risk of infection, including severe infections, vs other standard regimens due to antigen targeting of normal immune cells.3,6-8 CD38 is expressed on normal plasma cells, B cells, T cells, and natural killer cells, in addition to myeloma cells.8 BCMA is expressed on normal B cells and plasma cells, where it plays a role in B-cell maturation and plasma cell proliferation and survival, as well as on myeloma cells;3 BCMA-targeting therapies reduce the number of normal plasma cells and B cells, resulting in hypogammaglobulinemia and B-cell aplasia in some patients.3,9,10
G protein-coupled receptor class C group 5 member D (GPRC5D) is a novel target and orphan receptor in MM.11,12 Although GPRC5D and BCMA are both expressed on MM cells, relative to BCMA, GPRC5D has higher expression on malignant vs normal plasma cells, and is not as widely expressed on hematopoietic precursors.11,12 Otherwise, GPRC5D expression is mostly limited to nonhematologic, keratinized tissues such as hair follicles and filiform papillae of the tongue, with limited expression on normal B cells, T cells, natural killer cells, monocytes, granulocytes, bone marrow progenitors, and other healthy tissues.11-13
Talquetamab is a first-in-class, off-the-shelf, T-cell redirecting bispecific antibody directed against GPRC5D on MM cells and CD3 on T cells to induce killing of GPRC5D-expressing myeloma cells through T-cell recruitment and activation.13,14 Talquetamab has been approved by the US Food and Drug Administration and the European Medicines Agency for the treatment of patients with triple-class exposed relapsed/refractory MM (RRMM) who received ≥4 and ≥3 prior lines of therapy, respectively.15,16 Approval was based on results from the phase 1/2 MonumenTAL-1 study (NCT03399799/NCT04634552), which evaluated 2 recommended phase 2 doses (RP2Ds) of subcutaneous talquetamab (0.4 mg/kg weekly and 0.8 mg/kg every other week [EOW]) in patients naive to prior T-cell redirection therapy (TCR), and a separate cohort of patients who received prior TCR, that is, chimeric antigen receptor (CAR) T-cell or bispecific antibody therapies, at either dosing schedule.14-17 Overall response rates were 74.1% (weekly), 71.7% (EOW), and 64.7% (prior TCR), and both dose schedules had a clinically manageable safety profile. Most adverse events (AEs), including cytokine release syndrome, and skin, nail, and oral toxicities, were predominantly grade 1/2.17 The frequency of grade 3/4 infections with talquetamab (<28.0% across the 3 cohorts) was lower compared with BCMA-directed TCR.17-20 Here, we describe the infection profile and parameters of humoral immune function in patients receiving talquetamab in MonumenTAL-1.
Methods
Study design and patients
MonumenTAL-1 is a first-in-human, phase 1/2, open-label, multicenter study of talquetamab monotherapy. Detailed methods have been published previously.14 Briefly, the trial consists of a phase 1 dose escalation and expansion portion, which identified the 2 RP2Ds,14 and a phase 2 portion, which assessed the safety and efficacy of talquetamab at the RP2Ds. Eligible patients were aged ≥18 years, and had measurable myeloma per International Myeloma Working Group criteria. In phase 1, patients were required to have disease that had progressed on established therapies or an inability to tolerate them, and an Eastern Cooperative Oncology Group performance status of 0 or 1. In phase 2, patients were required to have ≥3 prior lines of therapy that included a proteasome inhibitor, immunomodulatory drug, and anti-CD38 antibody, and an Eastern Cooperative Oncology Group performance status of 0 to 2.
Treatment
Patients received talquetamab at the subcutaneous RP2Ds of 0.4 mg/kg weekly and 0.8 mg/kg EOW after 2 or 3 step-up doses, respectively. A glucocorticoid, an antihistamine, and paracetamol were given as premedications during the step-up dosing schedule to mitigate cytokine release syndrome, and patients were hospitalized for 48 hours from the start of step-up doses and the first full dose. Supportive care for infections, including oral and IV antibiotics and other antimicrobials, was administered according to standard institutional practice. Patients with serious infections included those who were hospitalized or experienced an important medical event or death. Immunoglobulin levels were monitored by AE reporting, laboratory analyses, or both, and hypogammaglobulinemia was treated according to local institutional guidelines. Vaccination with a live, attenuated vaccine was prohibited within 4 weeks before the first dose of talquetamab, and for 100 days after the last dose of talquetamab. Annual inactivated influenza and prophylactic COVID-19 vaccination were recommended where available, per the investigator’s discretion. COVID-19 vaccine response was assessed in a real-world patient population (supplemental Appendix 1).
Assessments
This was a post hoc analysis based on a data cut-off date of 17 January 2023. Descriptive statistics were generated for infection type, incidence, severity, timing, and management. Patients underwent frequent monitoring for the presence of infections, which were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03. CD19+ mature B cells were assessed from whole blood using flow cytometry. Blood samples were used for hematology laboratory tests, including hemoglobin, white blood cell count with differential, platelet count, absolute neutrophil count, and absolute lymphocyte count. GPRC5D protein expression in bone marrow samples was assessed by flow cytometry in a separate analysis of healthy donors and patients with MM (supplemental Appendix 1).
Results
Between 3 January 2018 and 20 February 2023, 735 patients were screened for enrollment across all phase 1 and 2 cohorts. As of 17 January 2023, 339 patients treated at the talquetamab RP2Ds were included in this analysis; 288 patients (143 receiving 0.4 mg/kg weekly and 145 receiving 0.8 mg/kg EOW) had no prior TCR exposure, and 51 patients (receiving either RP2D) had prior TCR exposure. Median follow-up was 18.8, 12.7, and 14.8 months in the weekly, EOW, and prior TCR cohorts, respectively. Median duration of talquetamab treatment was 6.9, 8.8, and 5.7 months, respectively. Baseline characteristics were similar across cohorts (supplemental Table 1).
Infections profile
Any-grade infections occurred in 58.7%, 66.2%, and 72.5% of patients in the weekly, EOW, and prior TCR cohorts, respectively; grade 3/4 infections were observed in 21.7%, 15.9%, and 27.5% of patients, respectively (Table 1). The most common grade 3/4 infections were pneumonia (3.5%) and urinary tract infections (2.1%) in the weekly cohort, COVID-19 (2.1%) and pneumonia (2.1%) in the EOW cohort, and pneumonia (5.9%) in the prior TCR cohort (Table 2). Median duration of infection was 11.5 days (range, 2-148 days), 12.0 days (range, 1-201 days), and 12.0 days (range, 2-51 days), respectively. Most infections resolved (94.0% in weekly, 93.8% in EOW, and 100% in prior TCR). The proportion of patients reporting recurrent, any-grade infections was 34.5% to 49.0% across cohorts. Three patients discontinued talquetamab due to infections, 2 in the weekly cohort (grade 3 pneumonia and grade 4 fungal sepsis [Candida]) and 1 in the prior TCR cohort (grade 3 pustular rash). In total, 5 patients (1.5%) died from infections (COVID-19 pneumonia, n = 2 [1 in weekly, 1 in EOW]: neither patient received COVID-19 vaccination; septic shock [1 in weekly], Candida sepsis [1 in weekly], and unknown etiology [1 in weekly]).
Summary of infection and cytopenia events
| . | 0.4 mg/kg weekly (n = 143) . | 0.8 mg/kg EOW (n = 145) . | Prior TCR (n = 51) . |
|---|---|---|---|
| Infections, n (%) | |||
| Any grade | 84 (58.7) | 96 (66.2) | 37 (72.5) |
| Grade 3/4 | 31 (21.7) | 23 (15.9) | 14 (27.5) |
| Led to death | 3 (2.1) | 2 (1.4) | 0 |
| Led to discontinuation | 2 (1.4) | 0 | 1 (2.0) |
| Led to dose interruption | 45 (31.5) | 49 (33.8) | 19 (37.3) |
| Neutropenia, n (%) | |||
| Any grade | 50 (35.0) | 41 (28.3) | 28 (54.9) |
| Grade 3/4 neutropenia | 44 (30.8) | 32 (22.1) | 27 (52.9) |
| Any infection + concomitant grade 3/4 neutropenia | 11 (7.7) | 3 (2.1) | 9 (17.6) |
| Grade 3/4 infection + concomitant grade 3/4 neutropenia | 4 (2.8) | 1 (0.7) | 1 (2.0) |
| Anemia, n (%) | |||
| Any grade | 64 (44.8) | 66 (45.5) | 25 (49.0) |
| Grade 3/4 | 45 (31.5)∗ | 40 (27.6)∗ | 14 (27.5)∗ |
| Lymphopenia, n (%) | |||
| Any grade | 40 (28.0) | 42 (29.0) | 9 (17.6) |
| Grade 3/4 | 37 (25.9) | 39 (26.9) | 7 (13.7) |
| Thrombocytopenia, n (%) | |||
| Any grade | 39 (27.3) | 43 (29.7) | 19 (37.3) |
| Grade 3/4 | 29 (20.3) | 27 (18.6) | 15 (29.4) |
| Leukopenia, n (%) | |||
| Any grade | 23 (16.1) | 27 (18.6) | 12 (23.5) |
| Grade 3/4 | 11 (7.7) | 18 (12.4) | 9 (17.6) |
| . | 0.4 mg/kg weekly (n = 143) . | 0.8 mg/kg EOW (n = 145) . | Prior TCR (n = 51) . |
|---|---|---|---|
| Infections, n (%) | |||
| Any grade | 84 (58.7) | 96 (66.2) | 37 (72.5) |
| Grade 3/4 | 31 (21.7) | 23 (15.9) | 14 (27.5) |
| Led to death | 3 (2.1) | 2 (1.4) | 0 |
| Led to discontinuation | 2 (1.4) | 0 | 1 (2.0) |
| Led to dose interruption | 45 (31.5) | 49 (33.8) | 19 (37.3) |
| Neutropenia, n (%) | |||
| Any grade | 50 (35.0) | 41 (28.3) | 28 (54.9) |
| Grade 3/4 neutropenia | 44 (30.8) | 32 (22.1) | 27 (52.9) |
| Any infection + concomitant grade 3/4 neutropenia | 11 (7.7) | 3 (2.1) | 9 (17.6) |
| Grade 3/4 infection + concomitant grade 3/4 neutropenia | 4 (2.8) | 1 (0.7) | 1 (2.0) |
| Anemia, n (%) | |||
| Any grade | 64 (44.8) | 66 (45.5) | 25 (49.0) |
| Grade 3/4 | 45 (31.5)∗ | 40 (27.6)∗ | 14 (27.5)∗ |
| Lymphopenia, n (%) | |||
| Any grade | 40 (28.0) | 42 (29.0) | 9 (17.6) |
| Grade 3/4 | 37 (25.9) | 39 (26.9) | 7 (13.7) |
| Thrombocytopenia, n (%) | |||
| Any grade | 39 (27.3) | 43 (29.7) | 19 (37.3) |
| Grade 3/4 | 29 (20.3) | 27 (18.6) | 15 (29.4) |
| Leukopenia, n (%) | |||
| Any grade | 23 (16.1) | 27 (18.6) | 12 (23.5) |
| Grade 3/4 | 11 (7.7) | 18 (12.4) | 9 (17.6) |
Percentages are calculated with the number of patients in the all-treated analysis set in each cohort as the denominator.
Grade 3 only.
Any-grade infection events in 4 or more patients and grade 3/4 infections in any cohort
| . | 0.4 mg/kg weekly (n = 143) . | 0.8 mg/kg EOW (n = 145) . | Prior TCR (n = 51) . |
|---|---|---|---|
| Infections | |||
| COVID-19 | 15 (10.5) | 34 (23.4) | 6 (11.8) |
| Upper respiratory tract infection | 18 (12.6) | 13 (9.0) | 9 (17.6) |
| Nasopharyngitis | 14 (9.8) | 10 (6.9) | 2 (3.9) |
| Urinary tract infection | 14 (9.8) | 6 (4.1) | 6 (11.8) |
| Pneumonia | 11 (7.7) | 9 (6.2) | 3 (5.9) |
| Bronchitis | 12 (8.4) | 6 (4.1) | 0 |
| Rhinovirus infection | 4 (2.8) | 6 (4.1) | 4 (7.8) |
| Respiratory tract infection | 6 (4.2) | 4 (2.8) | 3 (5.9) |
| Oral candidiasis | 5 (3.5) | 4 (2.8) | 2 (3.9) |
| Gastroenteritis | 5 (3.5) | 1 (0.7) | 2 (3.9) |
| Pharyngitis | 2 (1.4) | 4 (2.8) | 2 (3.9) |
| Sinusitis | 3 (2.1) | 4 (2.8) | 1 (2.0) |
| Rhinitis | 4 (2.8) | 1 (0.7) | 0 |
| Grade 3/4 infections | |||
| Pneumonia | 5 (3.5) | 3 (2.1) | 3 (5.9) |
| COVID-19 | 2 (1.4) | 3 (2.1) | 1 (2.0) |
| Urinary tract infection | 3 (2.1) | 0 | 2 (3.9) |
| COVID-19 pneumonia | 1 (0.7) | 2 (1.4) | 1 (2.0) |
| Sepsis | 2 (1.4) | 1 (0.7) | 0 |
| Cellulitis | 0 | 2 (1.4) | 0 |
| Infection (unknown etiology) | 0 | 2 (1.4) | 0 |
| Bronchiolitis | 1 (0.7) | 1 (0.7) | 0 |
| Device-related infection | 1 (0.7) | 1 (0.7) | 0 |
| Escherichia urinary tract infection | 1 (0.7) | 0 | 1 (2.0) |
| Respiratory syncytial virus | 1 (0.7) | 1 (0.7) | 0 |
| Septic shock | 1 (0.7) | 1 (0.7) | 0 |
| Sinusitis | 0 | 1 (0.7) | 1 (2.0) |
| Staphylococcus infection | 1 (0.7) | 1 (0.7) | 0 |
| Upper respiratory tract infection | 1 (0.7) | 1 (0.7) | 0 |
| Bacteremia | 1 (0.7) | 0 | 0 |
| Bacterial prostatitis | 1 (0.7) | 0 | 0 |
| Disseminated varicella zoster virus infection | 0 | 0 | 1 (2.0) |
| Diverticulitis | 0 | 1 (0.7) | 0 |
| Enterobacter bacteremia | 1 (0.7) | 0 | 0 |
| Escherichia sepsis | 0 | 0 | 1 (2.0) |
| Fungal sepsis | 1 (0.7) | 0 | 0 |
| Gastroenteritis, Escherichia coli | 1 (0.7) | 0 | 0 |
| Herpes zoster | 1 (0.7) | 0 | 0 |
| Human ehrlichiosis | 1 (0.7) | 0 | 0 |
| Implant site infection | 1 (0.7) | 0 | 0 |
| Influenza | 1 (0.7) | 0 | 0 |
| Klebsiella bacteremia | 1 (0.7) | 0 | 0 |
| Klebsiella sepsis | 0 | 1 (0.7) | 0 |
| Large intestine infection | 0 | 1 (0.7) | 0 |
| Metapneumovirus infection | 0 | 0 | 1 (2.0) |
| Esophageal candidiasis | 1 (0.7) | 0 | 0 |
| Parainfluenzae virus infection | 1 (0.7) | 0 | 0 |
| Pneumococcus sepsis | 0 | 0 | 1 (2.0) |
| Pneumonia influenza | 0 | 0 | 1 (2.0) |
| Pseudomonas bacteremia | 0 | 1 (0.7) | 0 |
| Rash pustular | 0 | 0 | 1 (2.0) |
| Respiratory tract infection | 0 | 1 (0.7) | 0 |
| Retinitis | 1 (0.7) | 0 | 0 |
| Rhinovirus infection | 1 (0.7) | 0 | 0 |
| Salmonella sepsis | 0 | 1 (0.7) | 0 |
| Skin infection | 0 | 1 (0.7) | 0 |
| Staphylococcus bacteremia | 0 | 1 (0.7) | 0 |
| Staphylococcus sepsis | 1 (0.7) | 0 | 0 |
| Streptococcus bacteremia | 1 (0.7) | 0 | 0 |
| Streptococcus pneumonia | 0 | 1 (0.7) | 0 |
| Urinary tract infection, Pseudomonas | 0 | 0 | 1 (2.0) |
| Vascular device infection | 0 | 0 | 1 (2.0) |
| . | 0.4 mg/kg weekly (n = 143) . | 0.8 mg/kg EOW (n = 145) . | Prior TCR (n = 51) . |
|---|---|---|---|
| Infections | |||
| COVID-19 | 15 (10.5) | 34 (23.4) | 6 (11.8) |
| Upper respiratory tract infection | 18 (12.6) | 13 (9.0) | 9 (17.6) |
| Nasopharyngitis | 14 (9.8) | 10 (6.9) | 2 (3.9) |
| Urinary tract infection | 14 (9.8) | 6 (4.1) | 6 (11.8) |
| Pneumonia | 11 (7.7) | 9 (6.2) | 3 (5.9) |
| Bronchitis | 12 (8.4) | 6 (4.1) | 0 |
| Rhinovirus infection | 4 (2.8) | 6 (4.1) | 4 (7.8) |
| Respiratory tract infection | 6 (4.2) | 4 (2.8) | 3 (5.9) |
| Oral candidiasis | 5 (3.5) | 4 (2.8) | 2 (3.9) |
| Gastroenteritis | 5 (3.5) | 1 (0.7) | 2 (3.9) |
| Pharyngitis | 2 (1.4) | 4 (2.8) | 2 (3.9) |
| Sinusitis | 3 (2.1) | 4 (2.8) | 1 (2.0) |
| Rhinitis | 4 (2.8) | 1 (0.7) | 0 |
| Grade 3/4 infections | |||
| Pneumonia | 5 (3.5) | 3 (2.1) | 3 (5.9) |
| COVID-19 | 2 (1.4) | 3 (2.1) | 1 (2.0) |
| Urinary tract infection | 3 (2.1) | 0 | 2 (3.9) |
| COVID-19 pneumonia | 1 (0.7) | 2 (1.4) | 1 (2.0) |
| Sepsis | 2 (1.4) | 1 (0.7) | 0 |
| Cellulitis | 0 | 2 (1.4) | 0 |
| Infection (unknown etiology) | 0 | 2 (1.4) | 0 |
| Bronchiolitis | 1 (0.7) | 1 (0.7) | 0 |
| Device-related infection | 1 (0.7) | 1 (0.7) | 0 |
| Escherichia urinary tract infection | 1 (0.7) | 0 | 1 (2.0) |
| Respiratory syncytial virus | 1 (0.7) | 1 (0.7) | 0 |
| Septic shock | 1 (0.7) | 1 (0.7) | 0 |
| Sinusitis | 0 | 1 (0.7) | 1 (2.0) |
| Staphylococcus infection | 1 (0.7) | 1 (0.7) | 0 |
| Upper respiratory tract infection | 1 (0.7) | 1 (0.7) | 0 |
| Bacteremia | 1 (0.7) | 0 | 0 |
| Bacterial prostatitis | 1 (0.7) | 0 | 0 |
| Disseminated varicella zoster virus infection | 0 | 0 | 1 (2.0) |
| Diverticulitis | 0 | 1 (0.7) | 0 |
| Enterobacter bacteremia | 1 (0.7) | 0 | 0 |
| Escherichia sepsis | 0 | 0 | 1 (2.0) |
| Fungal sepsis | 1 (0.7) | 0 | 0 |
| Gastroenteritis, Escherichia coli | 1 (0.7) | 0 | 0 |
| Herpes zoster | 1 (0.7) | 0 | 0 |
| Human ehrlichiosis | 1 (0.7) | 0 | 0 |
| Implant site infection | 1 (0.7) | 0 | 0 |
| Influenza | 1 (0.7) | 0 | 0 |
| Klebsiella bacteremia | 1 (0.7) | 0 | 0 |
| Klebsiella sepsis | 0 | 1 (0.7) | 0 |
| Large intestine infection | 0 | 1 (0.7) | 0 |
| Metapneumovirus infection | 0 | 0 | 1 (2.0) |
| Esophageal candidiasis | 1 (0.7) | 0 | 0 |
| Parainfluenzae virus infection | 1 (0.7) | 0 | 0 |
| Pneumococcus sepsis | 0 | 0 | 1 (2.0) |
| Pneumonia influenza | 0 | 0 | 1 (2.0) |
| Pseudomonas bacteremia | 0 | 1 (0.7) | 0 |
| Rash pustular | 0 | 0 | 1 (2.0) |
| Respiratory tract infection | 0 | 1 (0.7) | 0 |
| Retinitis | 1 (0.7) | 0 | 0 |
| Rhinovirus infection | 1 (0.7) | 0 | 0 |
| Salmonella sepsis | 0 | 1 (0.7) | 0 |
| Skin infection | 0 | 1 (0.7) | 0 |
| Staphylococcus bacteremia | 0 | 1 (0.7) | 0 |
| Staphylococcus sepsis | 1 (0.7) | 0 | 0 |
| Streptococcus bacteremia | 1 (0.7) | 0 | 0 |
| Streptococcus pneumonia | 0 | 1 (0.7) | 0 |
| Urinary tract infection, Pseudomonas | 0 | 0 | 1 (2.0) |
| Vascular device infection | 0 | 0 | 1 (2.0) |
Percentages are calculated with the number of patients in the all-treated analysis set in each cohort as the denominator.
The most common any-grade infections were respiratory infections, occurring in 34.3% (in weekly), 28.3% (in EOW), and 39.2% (in prior TCR) of patients; most common were upper respiratory tract infections (9.0%-17.6%) and COVID-19 (10.5%-23.4%) across cohorts (Table 2). The most common grade 3/4 infections were pneumonia (2.1%-5.9%) and urinary tract infections (0%-3.9%) across cohorts. There were few gastrointestinal infections throughout talquetamab treatment (3.5% in weekly, 2.1% in EOW, and 3.9% in prior TCR). Viral infections including adenovirus, herpes zoster, and oral herpes occurred in 2.1% (in QW), 2.8% (in Q2W), and 7.8% (in prior TCR), and fungal infections occurred in 16 (11.2%), 11 (7.6%), and 6 (11.8%) patients, respectively; most fungal infections were noninvasive.
Opportunistic infections occurred in 5 (3.5%), 8 (5.5%), and 3 (5.9%) patients in the weekly, EOW, and prior TCR cohort, respectively (supplemental Table 2). Across cohorts, 3 patients had grade ≥3 opportunistic infections (esophageal candidiasis and Candida sepsis in QW, and disseminated varicella-zoster virus infection in prior TCR). No cases of Pneumocystis pneumonia were reported. Data related to antimicrobial prophylaxis are discussed below.
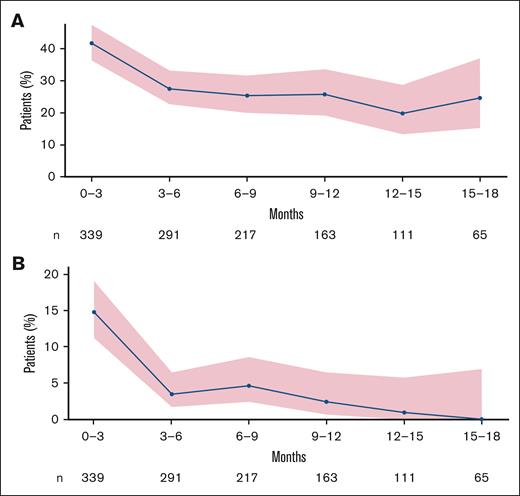
Any-grade infections were generally most common in cycles 1 and 2 (30.2% in weekly, 23.0% in EOW, and 45.1% in prior TCR; supplemental Table 3); bacterial and fungal infections tended to occur early in treatment (Figure 1). New-onset grade 3/4 infections were most prevalent during the first 100 days (Figure 1). Similarly, although risk of any-grade infection was highest during early cycles of talquetamab, risk declined at 3 months (Figure 2), and cumulative incidence of any-grade and grade ≥3 infections leveled off at ∼8 months and ∼4 months, respectively (supplemental Figure 1). Grade 3/4 infections were more common in nonresponders compared with responders (supplemental Figure 2).
Timing and severity of clinically relevant infections by location and etiology. (A) 0.4 mg/kg weekly cohort. (B) 0.8 mg/kg EOW cohort. (C) Prior TCR cohort. The y-axis (days) was the relative start date of the AE. Patients with multiple infections are represented as separate points on the plot. Patients included once on the plot are represented as circles, whereas patients included more than once are represented as triangles. Two patients were excluded from the location figures: 1 had a central nervous system event in the EOW cohort, and 1 had a dental event in the prior TCR cohort.
Timing and severity of clinically relevant infections by location and etiology. (A) 0.4 mg/kg weekly cohort. (B) 0.8 mg/kg EOW cohort. (C) Prior TCR cohort. The y-axis (days) was the relative start date of the AE. Patients with multiple infections are represented as separate points on the plot. Patients included once on the plot are represented as circles, whereas patients included more than once are represented as triangles. Two patients were excluded from the location figures: 1 had a central nervous system event in the EOW cohort, and 1 had a dental event in the prior TCR cohort.
Risk of infections over time across cohorts. (A) Any-grade infections. (B) Grade ≥3 infections.
Risk of infections over time across cohorts. (A) Any-grade infections. (B) Grade ≥3 infections.
Serious infections that led to overnight hospitalizations occurred in 15.4%, 9.7%, and 7.8% of patients in the weekly, EOW, and prior TCR cohorts, respectively, with a median duration of hospital stay of 8.0 days (range, 2-21 days), 5.5 days (range, 3-24 days), and 10.0 days (range, 4-25 days), respectively. Multiple medical encounters, including overnight hospitalizations, emergency room visits, or outpatient visits, due to infections occurred in 14 (9.8%), 8 (5.5%), and 2 (3.9%) patients, respectively.
Deaths
Deaths due to any cause occurred in 33.6%, 22.8%, and 39.2% of patients in the weekly, EOW, and prior TCR cohorts, respectively. Most deaths were due to progressive disease (19.6%, 17.2%, and 37.3%, respectively). AEs led to death in 3.5% (in weekly), 4.1% (in EOW), and 0% (in prior TCR) of patients. The number of infections that led to death was low (2.1%, 1.4%, and 0%, respectively). Despite enrollment during the global COVID pandemic, the number of deaths due to COVID-19 was low (COVID-19 pneumonia, n = 2 [1 in weekly, 1 in EOW]; no patients in the prior TCR cohort).
Cytopenias
The most common investigator-reported cytopenias of any grade were neutropenia (35.0% in weekly, 28.3% in EOW, and 54.9% in prior TCR) and anemia (44.8%, 45.5%, and 49.0%, respectively; Table 1). Any-grade lymphopenia occurred in 28.0%, 29.0%, and 17.6% of patients, respectively. Thrombocytopenia and leukopenia were observed less frequently across cohorts (Table 1). Hematologic toxicities were the most common grade 3/4 events. Infections occurred concurrently with grade 3/4 neutropenia in 7.7%, 2.1%, and 17.6% of patients in the 3 respective cohorts. Febrile neutropenia occurred in 2.8%, 0.7%, and 3.9% of patients, respectively (all grade 3/4).
Neutropenia occurred most frequently during step-up doses and cycle 1, with a median time to onset of 17, 12, and 8 days for grade ≥3 neutropenia across the respective cohorts. Median duration of grade 3/4 neutropenia events was 7, 8, and 7 days in the weekly, EOW, and prior TCR cohorts, respectively. Neutrophil counts decreased during cycle 1, but showed a steady recovery beginning at cycle 2 (Figure 3A). Similarly, hemoglobin levels and platelet counts showed an initial decline at cycle 1 that recovered at cycle 2 and were maintained through cycle 12 (Figure 3B-C). Lymphocyte counts also decreased during cycle 1, but recovered during cycle 2 and were maintained throughout treatment across cohorts (data not shown).
Cytopenias over time in the weeekly and EOW cohorts. (A) Neutrophil levels. (B) Platelet levels. (C) Hemoglobin levels. Data presented are based on laboratory values. SE, standard error.
Cytopenias over time in the weeekly and EOW cohorts. (A) Neutrophil levels. (B) Platelet levels. (C) Hemoglobin levels. Data presented are based on laboratory values. SE, standard error.
Humoral immune effects
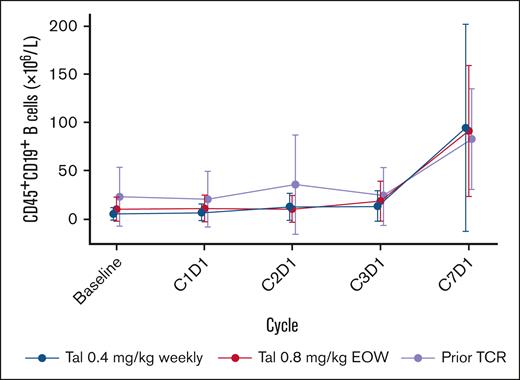
At baseline, median CD19+ B-cell counts were 5.3, 10.1, and 22.8 (×106 per L) in the weekly, EOW, and prior TCR cohorts, respectively. CD19+ B-cell frequencies remained stable throughout early treatment cycles, with an increasing trend noted at cycle 7 (Figure 4). Hypogammaglobulinemia (immunoglobulin G [IgG] values <400 mg/dL) at baseline was observed in 36.4% (in weekly), 31.7% (in EOW), and 49.0% (in prior TCR) of patients. After initiation of treatment, hypogammaglobulinemia was observed in 57.3%, 60.7%, and 66.7% of patients, respectively. Treatment-emergent hypogammaglobulinemia occurred in 23.1%, 28.3%, and 19.6% of patients, respectively. In alignment with these data, few patients received IV immunoglobulin before and after talquetamab treatment (discussed further below). Polyclonal IgG levels initially decreased from baseline to cycle 3 (3 months) in the combined cohorts (Figure 5), mostly due to patients in the prior TCR cohort, as levels in the weekly and EOW cohorts remained relatively stable and below the lower limit of normal (7.0 g/L) during this period (supplemental Figure 3). Polyclonal IgG levels in the overall population began to increase after cycle 3, and continued to increase above baseline levels through cycle 17 (17 months; Figure 5). Although measurable immunoglobulin A (IgA) and immunoglobulin M (IgM) samples were limited, polyclonal IgA and IgM levels were stable with no decrease from baseline through cycle 7 across all cohorts, and remained below the lower limit of normal (0.7 g/L for IgA and 0.4 g/L for IgM; supplemental Figures 4 and 5).
CD19+ B-cell levels from baseline through cycle 7 in all cohorts. Points represent the median B-cell count and whiskers show the median absolute deviation. At baseline, n = 52 in the qw cohort, n = 52 in the q2w cohort, and n = 18 in the prior TCR cohort.
CD19+ B-cell levels from baseline through cycle 7 in all cohorts. Points represent the median B-cell count and whiskers show the median absolute deviation. At baseline, n = 52 in the qw cohort, n = 52 in the q2w cohort, and n = 18 in the prior TCR cohort.
Mean polyclonal IgG adjusted cells during treatment. IgG was assessed monthly. The majority of samples collected were based on central laboratory testing. Polyclonal IgG was estimated for patients with IgG MM by subtracting M-spike protein values from total IgG values. These calculated values, along with measured IgG levels for patients with non-IgG MM, were assessed to derive mean IgG levels during treatment. Patients who received IV immunoglobulin before receiving talquetamab were included.
Mean polyclonal IgG adjusted cells during treatment. IgG was assessed monthly. The majority of samples collected were based on central laboratory testing. Polyclonal IgG was estimated for patients with IgG MM by subtracting M-spike protein values from total IgG values. These calculated values, along with measured IgG levels for patients with non-IgG MM, were assessed to derive mean IgG levels during treatment. Patients who received IV immunoglobulin before receiving talquetamab were included.
Infections management
Across cohorts, 14.7% (in weekly), 13.1% (in EOW), and 15.7% (in prior TCR) of patients received at least 1 dose of IV immunoglobulin, which included 4.9%, 6.2%, and 9.8%, respectively, who received IV immunoglobulin before starting talquetamab treatment, and 9.8%, 6.9%, and 5.9% of patients, respectively, who started IV immunoglobulin while on talquetamab treatment. Of the patients who had a serious infection while receiving talquetamab (18.9% in weekly, 15.9% in EOW, 19.6% in prior TCR), 40.7%, 13.0%, and 10.0%, respectively, subsequently received IV immunoglobulin.
Use of growth factors before starting talquetamab (7-day washout period required per protocol) was low across cohorts (3.5% in weekly, 1.4% in EOW, 3.9% in prior TCR). Throughout talquetamab treatment, growth factors were administered in 23.1%, 15.2%, and 37.3%, respectively.
Infection prophylaxis and management also included antiviral, antifungal, and antibiotic medications. The most commonly used prophylaxis was antiviral therapy (90.9% in weekly, 82.8% in EOW, 92.2% in prior TCR), with prevention of herpes infection reported as the most common reason for prophylaxis (88.8%, 82.8%, 90.2%, respectively). Among prophylactic medications, acyclovir was the most common, with use in 52.4%, 71.7%, and 74.5% of patients in each cohort, respectively. Valacyclovir was the second most common medication, with use in 37.1%,11.0%, and 17.6% of patients in each cohort, respectively. At baseline, 22.4% (in weekly), 24.1% (in EOW), and 31.4% (in prior TCR) of patients received prophylaxis for Pneumocystis jirovecii pneumonia; the most common regimen was the combination of sulfamethoxazole and trimethoprim. While on talquetamab treatment, 34.3%, 31.5%, and 43.1% of patients, respectively, received prophylaxis for P jirovecii pneumonia (including those who continued prophylaxis from baseline).
Supportive measures used to treat COVID-19 infection included anti-infectives (4.9% in weekly, 15.9% in EOW, 3.9% in prior TCR), glucocorticoids (1.4% in EOW only), and tocilizumab (0.7% in EOW only). Many patients received COVID-19 vaccination (59.4% in weekly, 62.8% in EOW, 41.2% in prior TCR); no patients received monoclonal antibodies for prophylaxis throughout treatment.
Real-world COVID-19 vaccination cohort
The serological response to the COVID-19 messenger RNA vaccination was evaluated in a real-world cohort that included a subset of patients treated with talquetamab (n = 14) or BCMA-directed bispecific antibodies (n = 16) from 1 study site (see supplemental Appendix 1 for methodology). Baseline characteristics for these patients are shown in supplemental Table 4. Patients treated with talquetamab developed high levels of antispike IgG antibodies (0-3 months after 2 doses, median 161.0 AU/mL; 0-3 months after 3 doses, median 1094.0 AU/mL) that were maintained, with higher levels observed with talquetamab compared with BCMA bispecific antibodies (any time point after 2 doses, median 113.0 vs 18.5 AU/mL, P = .027; any time point after 3 doses, median 478.0 vs 128.0 AU/mL, P = .0029; supplemental Figure 6).
GPRC5D expression in healthy patients vs patients with MM
In a separate analysis of healthy volunteers and patients with MM, GPRC5D expression was evaluated by flow cytometry analysis of bone marrow samples (see supplemental Appendix 1 for methodology). Results revealed that human hematopoietic stem cells lacked GPRC5D expression in both healthy controls and patients with MM. Expression of GPRC5D was not observed in lymphoid progenitors or in late pro-B cells in healthy controls, and was minimal in late pro-B cells in patients with MM. GPRC5D expression was observed in plasma cells of both healthy controls and patients with MM (supplemental Figure 7); GPRC5D expression levels were lower on normal vs malignant plasma cells.
Discussion
In this analysis of the infection and immune cell profile of patients treated with talquetamab in MonumenTAL-1, ∼20% of patients had grade 3/4 infections, with most occurring during cycles 1 and 2, which is consistent with a decline in grade ≥3 infection risk after 3 months of talquetamab treatment. Interestingly, nonresponders to talquetamab had higher grade 3/4 infections compared with responders, suggesting patients’ infection risk is, at least in part, related to their disease, not talquetamab treatment. Relatively low rates of opportunistic infections (4.7% in total), discontinuations (0.9% in total), and deaths (1.5% in total) due to infections were observed. Neutrophil, lymphocyte, and platelet counts, along with hemoglobin levels, showed an initial decline after talquetamab administration, but levels recovered quickly and returned close to baseline at cycle 2 and were maintained throughout treatment. No decreases in B cells and increases in polyclonal IgG levels were observed, and no negative impact on COVID-19 vaccination antispike antibody levels was seen with talquetamab. These data support talquetamab as a B-cell sparing treatment that allows preservation of key elements of humoral immunity, including normal plasma cell development, polyclonal immunoglobulin production, and antigen-specific immune response to vaccination.
Infection and cytopenia results were generally similar across talquetamab cohorts, with the exception of any-grade and grade 3/4 infections and neutropenia events, each of which showed a higher frequency in the prior TCR cohort compared with the weekly and EOW cohorts. Differences in baseline immune fitness could help explain these results, as translational data have shown trends for higher expression of inhibitory markers on CD8+ T cells (including expression of programmed cell death protein-1/lymphocyte-activation gene 3 [PD-1/LAG-3], cluster of differentiation 38 [CD38], and T-cell immunoglobulin and mucin domain-containing 3 [TIM-3]), and higher frequencies of regulatory T cells in the prior TCR cohort compared with weekly and EOW cohorts, indicating a less fit immune phenotype in patients with prior TCR, potentially due to more extensive treatment exposure as well as immune depletion as a result of BCMA-targeted TCR.21
The infection profile with talquetamab appears to be consistent with other GPRC5D-targeted agents in development, including forimtamig, a GPRC5D × CD3 bispecific antibody currently being evaluated in a phase 1 study,22 but distinct from BCMA-targeted bispecific antibodies.14,17,20,22 Although trial comparisons should be made with caution, grade 3/4 infections were ≤28% across the talquetamab cohorts (including patients with prior TCR) compared with rates of 40% and 55% with the BCMA-targeting bispecific antibodies elranatamab in the MagnetisMM-3 study (median follow-up, 14.7 months) and teclistamab in the MajesTEC-1 study (median follow-up, 22.8 months), respectively, in patients with RRMM (no prior BCMA-targeted therapies).20,23
Unlike BCMA, which is expressed on normal plasma cells and B cells, GPRC5D expression is restricted to malignant plasma cells with limited expression on other hematopoietic cells (supplemental Figures 7 and 8),24 which potentially explains the lower infection rates and shorter duration of infections seen with talquetamab.19,20,23 With BCMA-directed therapies, data have shown prolonged cytopenia with a longer recovery period after treatment (BCMA CAR T-cell therapy)25,26 and most frequent neutropenia at 2 to 4 months following treatment start with a median time to onset of ∼2 months for grade ≥3 events (teclistamab).20 Patients treated with talquetamab showed a quick recovery, and neutropenia occurred most frequently during early treatment cycles with a median time to onset of 8 to 17 days for grade ≥3 neutropenia across cohorts. This, along with lower rates of severe infection, suggests that talquetamab, with its unique targeting of GPRC5D, allows for a faster recovery of normal bone marrow and humoral function in patients with RRMM due to the minimal collateral impact on normal immune cells.
The timing of infections overall appears to be unique with talquetamab compared with BCMA-directed bispecific antibodies. With talquetamab, any-grade infections occurred mostly in early cycles, and grade 3/4 infections were mostly limited to the first 100 days. With BCMA-directed bispecific antibodies, infections are prevalent throughout treatment, but tend to plateau with management strategies including dose modifications and use of IV immunoglobulin.20 These data suggest that talquetamab may be a better treatment option in some patients who are at a higher risk of complications from infections, such as those with chronic obstructive pulmonary disease, asthma, or any other conditions that may make it difficult to recover from an infection.
A more favorable infections profile has implications for management of patients on talquetamab. First, there may be less need for aggressive monitoring or routine use of IV immunoglobulin (prophylaxis and management) compared with BCMA-directed therapies. Due to the potential B-cell aplasia with BCMA bispecific antibodies, IV immunoglobulin is administered more regularly to provide humoral protection and reduce the rate of severe infections. In the MajesTEC-1 study, it was recommended to administer IgG replacement every 3 to 6 weeks to maintain serum IgG ≥400 mg/dL, and monitor every 3 months until a steady state had been reached per the physician’s discretion; prophylactic IgG replacement was based on physician-assessed benefit.19,20 Lower rates of hypogammaglobulinemia were observed with talquetamab compared with other BCMA-directed therapies, which corresponded to fewer patients receiving at least 1 dose of IV immunoglobulin with talquetamab (14.7% in weekly, 13.1% in EOW, and 15.7% in prior TCR) compared with elranatamab (43.1%) and teclistamab (46.1%).20,23 Although further study is needed, this may be due to adequate control of MM with talquetamab together with preservation of B cells and polyclonal IgG (increased levels observed throughout treatment) and early recovery of cytopenias (observed starting at ∼cycle 2), allowing for more effective immune control and prevention of infections.
Second, more aggressive monitoring and preventive strategies including prophylaxis with growth factor support, IV immunoglobulin, and lifestyle considerations (masking and distancing from those who are ill) may be more appropriate during the first few cycles of talquetamab treatment, as the majority of new-onset infections occurred during cycles 1 and 2 of treatment. This would allow for optimal management earlier during the window of time before neutrophils and other blood cells have returned to levels closer to baseline.
The effects of myeloma therapies on immune function have an impact on infection risk that may manifest as an increase in hospitalization and associated costs. In addition, prolonged periods of infection, either high- or low-grade, as well as serial infections that increase the overall cumulative symptom burden, may have effects on overall fitness and quality of life. A heavy infection burden throughout the course of treatment may result in decreased fitness at the time of treatment progression, potentially reducing options for subsequent therapies such as CAR T-cell therapy, transplant, or other immunosuppressive therapies. By allowing for myeloma control concurrent with immune restoration, including robust antigen-specific immune responses to vaccines, talquetamab may better sustain fitness for subsequent treatment options with novel agents using T cells to target BCMA or other myeloma antigens,27 potentially resulting in prolongation of overall survival. Further investigation is warranted to determine the impact of infections on healthcare utilization, quality of life, and subsequent treatment outcomes in this population.
A therapy with a more manageable infection profile may also be a good combination partner in MM, given that several therapies are associated with increased risks of infection. Combination studies with talquetamab are currently ongoing, with early results suggesting no additive toxicities including infection risk.28,29
Limitations of MonumenTAL-1 included that it was a single-arm study: the lack of a placebo arm makes it difficult to distinguish between infection risk due to the disease course or due to talquetamab treatment, and the lack of competitor arms prohibits comparisons of infection risk between treatment regimens. In addition, based on differences in practices across institutions involved in MonumenTAL-1, correlation with outcomes in centers utilizing varying institutional standards for infection prevention and management may be challenging.
In conclusion, patients treated with talquetamab showed effective myeloma control with concurrent preservation of humoral immune function, recovery of cytopenias, low rates of grade 3/4 and opportunistic infections, and a low occurrence of discontinuations and deaths due to infections, distinguishing talquetamab as an important novel treatment option for patients with RRMM.
Acknowledgments
The authors thank the patients who participated in the study, and their families and caregivers, the physicians and nurses who cared for patients and supported this clinical trial, the staff members at the study sites, and those involved in data collection and analyses. The authors also thank Chalmer Tomlinson for his work on data analysis and visualization. Medical writing support was provided by Alana Dorfstatter and Tim Connelly of Eloquent Scientific Solutions, and was funded by Johnson & Johnson.
The MonumenTAL-1 study was funded by Johnson & Johnson.
Authorship
Contribution: C.S., N.W.C.J.v.d.D., B.L., N.L., L.R., S.P., O.V.O., and A.C. contributed to the study design, study conduct, and data analysis and interpretation; D.V., S.S., and D.C.-S. contributed to data acquisition, analysis, and interpretation; B.H., T.M., M.C., S.K., T.R., J.T., C.K., K.S.G., I.S., and C.H. contributed to the study design, study conduct, and data acquisition and interpretation; all authors participated in drafting or revising the manuscript, and all approved the final version for submission; and all authors had full access to all of the data in the study and accept full responsibility for the decision to submit for publication.
Conflict-of-interest disclosure: C.S. reports honoraria from MJH Life Sciences, OncLive, and Pfizer; serves on an advisory board for Johnson & Johnson; and reports consulting fees from Johnson & Johnson. N.W.C.J.v.d.D. reports research support from Amgen, Bristol Myers Squibb (BMS), Celgene, Novartis, Cellectis, and Johnson & Johnson; and serves on advisory boards for AbbVie, Adaptive, Amgen, Bayer, BMS, Celgene, Johnson & Johnson, Kite Pharma, Merck, Novartis, Oncopeptides, Pfizer, Roche, Sanofi, Servier, and Takeda, all paid to institution. B.L. has served on an advisory board for AbbVie, GlaxoSmithKline (GSK), Karyopharm, Johnson & Johnson, Pfizer, and Sanofi; and reports consulting fees from AbbVie, GSK, Karyopharm, Johnson & Johnson, Pfizer, and Sanofi. L.R. reports honoraria from BMS, GSK, Johnson & Johnson, Pfizer, Roche, and Sanofi; is a member of a board of directors or advisory committee for Amgen, BMS, Johnson & Johnson, Pfizer, and Sanofi; is a consultant for Amgen, BMS, GSK, Johnson & Johnson, Pfizer, and Sanofi; and reports research funding from BMS and Skyline Dx. D.V., S.S., D.C.-S., R.V., B.H., T.M., M.C., S.K., T.R., J.T., C.K., K.S.G., I.S., and C.H. are employees of Johnson & Johnson, and may hold stock, stock options, or equity in Johnson & Johnson. A.C. is a consultant for AbbVie, Adaptive, Amgen, Antengene, BMS, Forus, Genetech/Roche, GSK, Johnson & Johnson, Karyopharm, Millenium/Takeda, Sanofi/Genzyme; and reports research funding from Johnson & Johnson. The remaining authors declare no competing financial interests.
Correspondence: Carolina Schinke, Myeloma Center, University of Arkansas for Medical Sciences Winthrop P. Rockefeller Institute, 449 Jack Stephens Dr, Little Rock, AR 72205; email: CDSchinke@usma.edu.
References
Author notes
The data sharing policy of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparancy. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access Project site at http://yoda.yale.edu.
The full-text version of this article contains a data supplement.