Visual Abstract
TO THE EDITOR:
Aplastic anemia (AA) is a disease of immune-mediated hematopoietic stem cell destruction, in which T-cell hyperfunction and abnormal immune tolerance play imperative roles.1,2 Recent evidence suggests the potential of thrombopoietin-receptor agonists in restoring hematopoiesis in severe AA (SAA) by stimulating hematopoietic stem cells.3-5 Eltrombopag, an oral thrombopoietin-receptor agonist, can restore multilineage hematopoiesis6,7 in patients with SAA refractory or intolerant to immunosuppressive therapy (IST). Combining standard IST (horse antithymocyte globulin [ATG] + cyclosporine A [CsA]) with eltrombopag as first-line treatment has demonstrated higher response rates than standard IST, and 6-month response rates with rabbit ATG + CsA range from 33% to 57%.8-10 However, administering eltrombopag with ATG may cause hepatic dysfunction.8
Romiplostim, a thrombopoietin mimetic protein that enhances platelet production, improved hematopoietic function from around 1 month after starting treatment in ∼70% of patients with SAA or transfusion-dependent patients with AA ineligible for ATG treatment, eventually leading to transfusion independence or decreased transfusion requirements.11 Additionally, high-dose romiplostim was highly effective in eltrombopag-refractory patients with AA, and it has been suggested that sequential therapy with eltrombopag followed by romiplostim may further improve prognosis in patients with AA refractory to conventional therapy.12
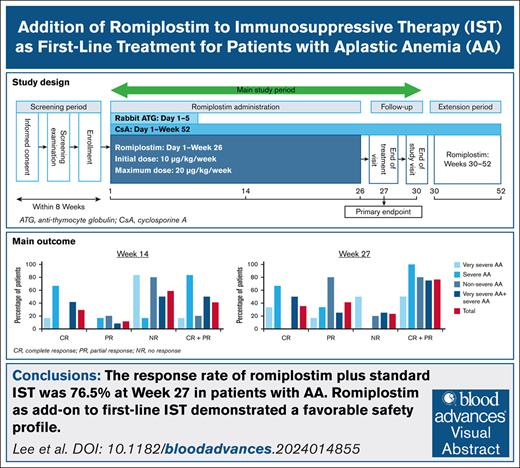
The current phase 2/3 study aimed to demonstrate the efficacy and safety of adding romiplostim to IST for patients with AA previously untreated with IST as a first-line treatment.
This open-label, phase 2/3 study included patients from Japan, South Korea, and Taiwan. The target population comprised adults with AA previously untreated with IST. Additional inclusion and exclusion criteria are described in the supplemental Methods. Institutional review boards approved the study, which followed the principles of the Declaration of Helsinki and Good Clinical Practice. All patients gave written informed consent. This clinical trial is registered with ClinicalTrials.gov under identifier NCT03957694. The study schedule and romiplostim administration are described in the supplemental Methods.
The primary efficacy end point was hematological response at week 27 with the achievement of complete response (CR) and partial response (PR) (based on the 531-003 response assessment criteria; supplemental Table 1). Secondary efficacy end points are described in the supplemental Methods. Safety outcomes for the safety analysis set included treatment-emergent adverse events (TEAEs), drug-related TEAEs, grade ≥3 TEAEs, and serious TEAEs. Other safety outcomes and the statistical methods are described in the supplemental Methods.
Patient disposition and characteristics at enrollment are shown in supplemental Figure 1 and supplemental Table 2. In the total population, the hematological response at week 14 was 41.2% (7/17 patients; 95% confidence interval [CI], 18.4-67.1; CR, 29.4% [5/17 patients]; PR, 11.8% [2/17 patients]) and 76.5% (13/17 patients; 95% CI, 50.1-93.2) at week 27 (CR, 35.3% [6/17 patients]; PR, 41.2% [7/17 patients]) (Table 1). Response rates by AA severity subgroup and by baseline reticulocyte count and age are shown in supplemental Tables 3 and 4. Median (range) changes from baseline in platelet count, hemoglobin concentration, reticulocyte count, and neutrophil count at week 27 were 128 × 103/μL (0 to 257 × 103/μL), 2.95 (−0.2 to 8.6) g/dL, 50.646 × 103/μL (19.843 × 103/μL to 87.780 × 103/μL), and 1.186 × 103/μL (.079 × 103/μL to 4.142 × 103/μL), respectively (all n = 17; Figure 1A-D). Responses in patients who continued romiplostim and entered the extended treatment period are shown in supplemental Table 5.
Hematologic response of the overall population at week 14 and at week 27 (full analysis set)
| Hematological response (n = 17) . | Response assessment at week 14 . | Response assessment at week 27 . |
|---|---|---|
| CR | 5 (29.4) | 6 (35.3) |
| PR | 2 (11.8) | 7 (41.2) |
| NR | 10 (58.8) | 4 (23.5) |
| Overall response (CR + PR) | 7 (41.2) | 13 (76.5) |
| 95% CI∗ | (18.4-67.1) | (50.1-93.2) |
| Hematological response (n = 17) . | Response assessment at week 14 . | Response assessment at week 27 . |
|---|---|---|
| CR | 5 (29.4) | 6 (35.3) |
| PR | 2 (11.8) | 7 (41.2) |
| NR | 10 (58.8) | 4 (23.5) |
| Overall response (CR + PR) | 7 (41.2) | 13 (76.5) |
| 95% CI∗ | (18.4-67.1) | (50.1-93.2) |
Data are number (%) unless otherwise stated.
NR, no response.
Calculated based on the Clopper–Pearson method.
Change from baseline to week 27 in blood count measurements. Mean (A) platelet count, (B) hemoglobin concentration, (C) reticulocyte count, and (D) neutrophil count until week 27 in the overall population. The platelet count obtained within 7 days after platelet transfusion was handled as missing. Hemoglobin concentration data obtained within 28 days after red blood cell transfusion were handled as missing. The neutrophil count obtained within 7 days after administration of granulocyte-colony stimulating factor was handled as missing.
Change from baseline to week 27 in blood count measurements. Mean (A) platelet count, (B) hemoglobin concentration, (C) reticulocyte count, and (D) neutrophil count until week 27 in the overall population. The platelet count obtained within 7 days after platelet transfusion was handled as missing. Hemoglobin concentration data obtained within 28 days after red blood cell transfusion were handled as missing. The neutrophil count obtained within 7 days after administration of granulocyte-colony stimulating factor was handled as missing.
All 17 patients experienced at least 1 TEAE, and 5 (29.4%) patients experienced at least 1 drug-related TEAE; constipation and headache were the most common AEs (supplemental Table 6). The most frequently reported drug-related TEAE was an increase in gamma-glutamyl transferase (2 patients [11.8%]); other drug-related TEAEs occurred in 1 (5.9%) patient each. No grade ≥3 drug-related TEAEs were reported. One (5.9%) patient discontinued because of a fatal TEAE of pneumonia (not considered related to romiplostim). No other TEAEs led to discontinuation of romiplostim and there were no fatal TEAEs. One additional patient developed a grade 1 reticulin elevation at week 53 that was considered by the investigators to be a romiplostim-related TEAE. No chromosomal abnormality was detected in karyotype analysis until week 27. No transformation to acute myeloid leukemia or myelodysplastic syndrome was observed.
Additional treatment options for AA are needed, as the efficacy of IST is limited to 70%, even with the addition of eltrombopag.8 This study evaluated the efficacy and safety of standard IST combined with romiplostim as a first-line therapy for AA. The efficacy of romiplostim was established by the primary end point of response based on the response assessment criteria, with an overall response rate of 76.5% and the lower limit of the 95% CI (50.1-93.2) exceeding the predetermined threshold. Efficacy was also supported by the secondary end points. Of the 11 patients who received romiplostim until the end of the extended treatment period, 9 had a response (CR or PR) at 53 weeks. Five patients developed drug-related TEAEs; none were grade ≥3 or resulted in death. No other significant TEAE was reported.
This is the first study of romiplostim used concomitantly with rabbit ATG and CsA in patients with AA previously untreated with IST. Although the response rate in the present study was similar to that of the eltrombopag study,13 which included 7 patients with SAA and 3 with nonsevere AA, the present study included 6 patients with very severe AA, suggesting that 10 to 20 μg/kg romiplostim per week is at least as active as 100 mg per day eltrombopag when used in combination with ATG + CsA for AA treatment.
The present findings are in concordance with other published studies of eltrombopag added to horse ATG + CsA in SAA.8,9 A phase 3 trial of previously untreated patients with SAA receiving horse ATG + CsA and eltrombopag (150 mg per day) had higher CR at 3 months (22% vs 10%) and 6 months (68% vs 41%) compared with those without eltrombopag. Eltrombopag was started on day 14 of ATG because of the potential risk of hepatic dysfunction.8,9 In the present study, romiplostim was started on day 1 of rabbit ATG therapy, based on the lower liver toxicity, and the response rate was similar to the response rate of the RACE study, in which eltrombopag 150 mg per day was started on day 14 of horse ATG. Given that rabbit ATG was associated with a significantly lower response rate than horse ATG,14 starting romiplostim on day 1 of ATG followed by a stepwise dose-escalation to the maximum dose (20 μg/kg per week) may be more effective than the delayed eltrombopag initiation.
The regimen used in the present phase 2/3 study produced a higher hematological response rate by week 27 (76.5%; 95% CI, 50.1-93.2) compared to the historical patients who received rabbit ATG + CsA (∼50% at 6 months),15 with manageable AEs.
A major limitation of the present study is the inclusion of both patients with SAA and nonsevere AA. Other limitations include the small sample size and limited follow-up period. Additionally, the enrolled patients were only of Asian origin, restricting generalizability to different populations. This was a single-arm study, limiting the evidence of response using romiplostim. Moreover, as the current study used rabbit ATG rather than horse ATG, future studies are needed to confirm the efficacy and safety of the combination of horse ATG, CsA, and romiplostim.
Overall, this phase 2/3 study demonstrated a favorable safety profile and efficacy of romiplostim in combination with ATG and CsA in adult patients with AA previously untreated with IST. The triple regimen of ATG and CsA with romiplostim might offer a new first-line treatment option in such patients.
Acknowledgments: The authors thank Aafreen Saiyed of Edanz for providing medical writing support, which was funded by Kyowa Kirin Co, Ltd, in accordance with Good Publication Practice 2022 guidelines (https://www.ismpp.org/gpp-2022). This study was funded by Kyowa Kirin Co, Ltd.
The funder of the study was involved in the study design, data collection, analysis, and interpretation.
Contributions: J.W.L., J.H.J., J.-L.T., H.C., M.N., K.M., and S.N. contributed to the study design; J.W.L., J.H.J., H.Y., M.S., M.K., Y.T., K.N., K.U., J.-P.G., and Y.M. collected data for the study; J.W.L., J.H.J., M.N., A.M., K.O., K.M., Y.K., and S.N. participated in the analysis and interpretation of the data and writing the manuscript; M.N. has accessed and verified the data; J.H.J. had final responsibility for the decision to submit for publication; and all authors reviewed the manuscript, approved the final version, and support this publication, and had full access to all data in the study and decided to submit the manuscript for publication.
Conflict-of-interest disclosure: J.W.L. reports consulting fees from Alexion Pharmaceuticals, Inc, Novartis Pharma K.K., Sanofi K.K., and Kyowa Kirin Co, Ltd. H.Y. reports payment or honoraria from Kyowa Kirin Co, Ltd, Novartis Pharma K.K., and Pfizer Japan Inc. M.S. reports payment or honoraria from Kyowa Kirin Co, Ltd. M.K. reports payment or honoraria from Bristol Myers Squibb K.K., Novartis Pharma K.K., Janssen Pharmaceutical K.K., Nippon Shinyaku Co, Ltd, Takeda Pharmaceutical Co, Ltd, and Ono Pharmaceutical Co, Ltd. Y.T. reports consulting fees from Kyowa Kirin Co, Ltd, Kissei Pharmaceutical Co, Ltd, Novartis Pharma K.K., and Swedish Orphan Biovitrum Japan Co, Ltd; payment or honoraria from Kyowa Kirin Co, Ltd, Kissei Pharmaceutical Co, Ltd, and Novartis Pharma K.K.; and payment for expert testimony from Swedish Orphan Biovitrum Japan Co, Ltd. K.U. reports grants or contracts from Astellas Pharma Inc, AbbVie G.K., Bristol Myers Squibb K.K., Janssen Pharmaceutical K.K., Ono Pharmaceutical Co, Ltd, Otsuka Pharmaceutical Co, Ltd, Chugai Pharmaceutical Co, Ltd, Apellis Pharmaceuticals, Inc, Yakult Honsha Co, Ltd, Merck Sharp & Dohme K.K., Amgen K.K., Alexion Pharmaceuticals, Inc, Incyte Biosciences Japan G.K., Eisai Co, Ltd, Kyowa Kirin Co, Ltd, Sanofi K.K., SymBio Pharmaceuticals Ltd, Celgene K.K., Daiichi Sankyo Co, Ltd, Sumitomo Pharma Co, Ltd, Nippon Shinyaku Co, Ltd, Novartis Pharma K.K., Mundipharma K.K., and Takeda Pharmaceutical Co, Ltd; consulting fees from Astellas Pharma Inc, Amgen K.K., Alnylam Japan K.K., Alexion Pharmaceuticals, Inc, Eisai Co, Ltd, Otsuka Pharmaceutical Co, Ltd, Ohara Pharmaceutical Co, Ltd, Kyowa Kirin Co, Ltd, Sanofi K.K., Sandoz K.K., SymBio Pharmaceuticals Ltd, Takeda Pharmaceutical Co, Ltd, Chugai Pharmaceutical Co, Ltd, and Nippon Shinyaku Co, Ltd; and payment or honoraria from Novartis Pharma K.K., AbbVie G.K., Alexion Pharmaceuticals, Inc, Incyte Biosciences Japan G.K., Ono Pharmaceutical Co, Ltd, Kyowa Kirin Co, Ltd, Sanofi K.K., Takeda Pharmaceutical Co, Ltd, Nippon Shinyaku Co, Ltd, Pfizer Japan Inc, and Bristol Myers Squibb K.K. A.M. reports consulting fees from Kyowa Kirin Co, Ltd; payment or honoraria from Novartis Pharma K.K., Sumitomo Pharma Co, Ltd, Alexion Pharmaceuticals, Inc, and Nippon Shinyaku Co, Ltd; and participation on a data safety monitoring board or advisory board for Kyowa Kirin Co, Ltd. K.M. reports grants or contracts from Kyowa Kirin Co, Ltd, and Chugai Pharmaceutical Co, Ltd; consulting fees from Kyowa Kirin Co, Ltd, Bristol Myers Squibb K.K.; payment or honoraria from Kyowa Kirin Co, Ltd, Chugai Pharmaceutical Co, Ltd, Novartis Pharma K.K., Bristol Myers Squibb K.K., and Sanofi K.K.; support for attending meetings and/or travel from Kyowa Kirin Co, Ltd; and participation on a data safety monitoring board or advisory board for Novartis Pharma K.K., and Bristol Myers Squibb K.K. Y.K. reports grants or contracts from Kyowa Kirin Co, Ltd; and payment or honoraria from Pfizer Japan Inc, Novartis Pharma K.K., and Sanofi K.K. S.N. reports payment or honoraria from Kyowa Kirin Co, Ltd. M.N. is an employee of Kyowa Kirin Co, Ltd. The remaining authors declare no competing financial interests.
Correspondence: Jun Ho Jang, Division of Hematology-Oncology, Sungkyunkwan University School of Medicine, Samsung Medical Center, 81 Irwon-Ro, Gangnam-Gu, Seoul 06351, Republic of Korea; email: jh21.jang@samsung.com.
References
Author notes
Presented in part at the European Hematology Association Hybrid Congress 2022, Vienna, Austria, 9 to 17 June 2022.
The data sets generated and/or analyzed will be available in the Vivli repository (available at: https://vivli.org/ourmember/kyowa-kirin/), as long as conditions of data disclosure specified in the policy section of the Vivli website are satisfied.
The full-text version of this article contains a data supplement.