Key Points
POD post–CAR-T for R/R MCL is associated with short OS and time from CAR-T infusion to POD predicted inferior OS in this setting.
Bispecific antibodies and combination small molecule approaches have activity in treating progressive disease post–CAR-T for R/R MCL.
Visual Abstract
The treatment patterns and clinical outcomes for patients experiencing progression of disease (POD) following CD19-directed chimeric antigen receptor (CAR) T-cell therapy for relapsed or refractory (R/R) mantle cell lymphoma (MCL) are undefined. We identified all patients who received CD19-directed CAR T-cell therapy for R/R MCL therapy across 15 international centers, and studied those experiencing POD post–CAR T-cell therapy in detail. We extracted clinical/treatment/pathologic variables, and associated these features with survival outcomes. In total, 384 patients received CAR T-cell therapy, and 135 (35%) experienced POD. POD occurred at a median of 6 months following CAR T-cell therapy infusion, and most (64%) patients with POD had complete response as best response to CAR T-cell therapy. Tumor features at POD included blastoid/pleomorphic morphology in 29 of 78 (37%) patients, and TP53 mutation in 21 of 41 (51%) patients. Following POD, 17 patients received no further therapy, 13 underwent local therapy, and 105 received systemic therapy. The most common first-line systemic therapies were chemo(immuno)therapy (22 patients; overall response rate [ORR], 40%), pirtobrutinib (17 patients; ORR, 36%), and bispecific antibodies (13 patients; ORR, 67%). Among patients experiencing POD, the median progression-free survival and overall survival (OS) were 2.5 months and 5.4 months, respectively, from POD. Lack of response to CAR T-cell therapy and short time from CAR T-cell therapy infusion to POD (<3 vs 3-6 vs >6 months), among other factors, were associated with inferior OS after POD. In conclusion, we confirm the challenging prognosis for patients experiencing POD following CD19 CAR T-cell therapy for R/R MCL, and establish a benchmark for future investigations in this patient population.
Introduction
Relapsed or refractory (R/R) mantle cell lymphoma (MCL) is frequently characterized by resistance to therapies, disease transformation, and short survival.1 CD19-directed chimeric antigen receptor (CAR) T-cell therapies are active in treating R/R MCL that progress after treatment with a covalent Bruton tyrosine kinase inhibitor (BTKi).2-4 Brexucabtagene autoleucel (brexu-cel), approved by the United States Food and Drug Administration in 2020, is associated with an overall response rate (ORR) of 91%, and a median progression-free survival (PFS) of 25.8 months. Lisocabtagene maraleucel (liso-cel), approved by the United States Food and Drug Administration in 2024, is associated with an ORR of 83%, and a median PFS 15.3 of months. Although highly active, brexu-cel and liso-cel do not appear curative, with evidence of a continuous pattern of relapse over time.
The management and survival outcomes of patients with R/R MCL experiencing progression of disease (POD) following CAR T-cell therapy remains largely undefined.5 In the only published experience, Jain and colleagues reported poor survival outcomes among 6 patients with R/R MCL with POD post–CAR T-cell therapy. Defining current outcomes post–CAR T-cell therapy failure would establish a critical benchmark for future trials investigating new therapies specifically for this patient population. A number of therapeutic options are available or under investigation for the treatment of R/R MCL, but their efficacy specifically post–CAR T-cell therapy is not known. For example, a phase 1/2 study of the CD20-CD3-directed bispecific antibody (BsAb) glofitamab demonstrated promising efficacy in R/R MCL with an ORR of 85%; however, only 2 of 60 treated patients had previously received CAR T-cell therapy.6 Here, we report results from a multinational, multicenter retrospective study investigating treatment patterns and clinical outcomes following POD post–CAR T-cell therapy for R/R MCL.
Methods
We queried institutional databases at each center to identify all patients with R/R MCL who had received CD19-directed CAR T-cell therapy. Treatment may have occurred as standard-of-care with an approved agent or in a clinical trial. For patients experiencing POD, we further investigated their clinical course with manual chart review performed by investigators at each institution to extract variables of interest. Site investigators performed response assessments according to institutional practices with available information. We defined bridging therapy as any therapy received between CAR T-cell therapy leukapheresis and lymphodepleting chemotherapy, regardless of previous receipt of that same therapy. We categorized therapies administered at POD according to mechanism(s) of action.
We used descriptive statistics to characterize patient/disease characteristics and treatment patterns. We used the reverse Kaplan-Meier method to calculate follow-up time from time of CAR T-cell therapy infusion, and the Kaplan-Meier method to calculate survival time from POD. We defined PFS as time from POD to subsequent POD or death, and overall survival (OS) as time from POD to death. For specific systemic therapy categories, we defined PFS as time from treatment initiation to disease progression or death, and duration of response (DOR) as time from treatment initiation to disease progression (among patients whose disease was responsive). We used Cox proportional hazards to associate patient/disease features with PFS/OS. R version 4.4.2 (R company) was used for all statistical analyses.7
We obtained institutional approval and secured data transfer agreements at all 15 sites for the purposes of this study. Deidentified data from each site were collated and securely stored at the institution of the corresponding author.
Results
Across 15 sites with 384 total patients who received CD19-directed CAR T-cell therapy for R/R MCL, we identified 135 (35%) who experienced POD by time of data collection, and who were investigated in detail and are elaborated upon (Table 1). The median follow-up for all patients with MCL receiving CAR T-cell therapy (ie, including those not experiencing POD at time of data cut-off) was available for patients from 14 of 15 sites (374/384 patients), and was 25 months (95% confidence interval [CI], 23, 28).
Patients with POD post–CAR T-cell therapy were mostly men (74%), with a median age of 67 years (interquartile range, 61-72) at time of CAR T-cell therapy infusion, and had received a median of 3 lines of therapy before leukapheresis (range, 1-9). Ninety-eight percent of patients received BTKi pre–CAR T-cell therapy, of whom 63% had disease response to BTKi. High-risk tumor features were common pre–CAR T-cell therapy: 54 of 79 (68%) with TP53 mutation, 79 of 114 (69%) with Ki67 ≥50%, 22 (16%) with central nervous system (CNS) involvement (at any time pre–CAR T-cell therapy), and 34 (25%) with blastoid/pleomorphic MCL. Most patients (73%) received bridging therapy, and 60% (47/78) of response-assessed patients’ disease was refractory to bridging. Patients received brexu-cel (87%) or liso-cel (13%) at the discretion of the treating physician, predominantly with fludarabine/cyclophosphamide lymphodepletion (87%).
Regarding best response to CAR T-cell therapy for patients with POD, 64% had a complete response, 27% partial response, and 16% had stable disease or POD. The median time from CAR T-cell therapy infusion to POD was 6 months (interquartile range, 3-11), and only 30 patients experienced POD >12 months from CAR T-cell therapy infusion. At the time of POD, high-risk MCL features were again common (Table 2; supplemental Table 3): 29 of 78 (37%) with blastoid/pleomorphic morphology, 50 of 65 (77%) with Ki67 ≥50%, and 21 of 41 (51%) with TP53-mutated MCL. Eleven of 22 patients with CNS disease pre–CAR T-cell therapy had CNS involvement at the time of POD. Among 79 patients with CD19 status assessed on a post-POD biopsy, 8 (10%) had CD19– disease. Twenty-nine percent of patients had an Eastern Cooperative Oncology Group performance status of ≥2 at time of POD.
Following POD, at time of data collection, 17 patients received no further lymphoma-directed therapy, 13 patients initially underwent local therapy with excision or radiation (4 such patients later received systemic therapy), and 105 patients initially received systemic therapy (Table 3; supplemental Table 1). The 4 patients whose first lymphoma-directed therapy was localized and later received systemic treatment are included in the subsequent discussion. The most commonly used first-line systemic therapies following CAR T-cell therapy included: chemo(immuno)therapy in 22 patients (ORR, 40%), pirtobrutinib in 17 patients (ORR, 36%), and BsAb in 13 patients (ORR, 67%). For patients treated with BsAb, the median time from CAR T-cell therapy to BsAb initiation was 10.5 months (range, 1.8-29.8 months); the median DOR was 10.5 months, median PFS 8.1 months, and median OS 8.3 months (supplemental Figure 1). A final noteworthy category of systemic treatments was small molecule inhibitor doublets (composition of doublets detailed in Table 3), associated with an ORR of 63% (5/8 patients; see Table 3 for therapy details). The ORR to all first systemic therapy after POD was 44%.
Eight patients underwent allogeneic hematopoietic cell transplant (allo-HCT) as response consolidation following subsequent therapies after POD: 3 following 1 line of therapy, 4 following 2 lines of therapy, and 1 following 3 lines of therapy. Considering these 8 patients, the median age at POD was 59 years (range, 40-68 years), and 6 are alive without disease, 1 died from graft-versus-host-disease, and 1 had disease relapse and is alive receiving subsequent therapy (median follow-up from allo-HCT: 9 months, range 1-22 months).
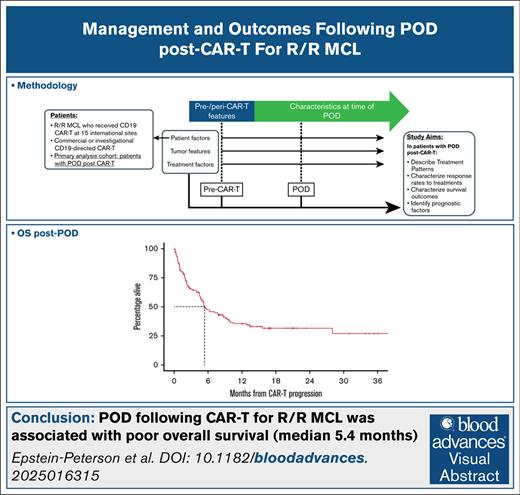
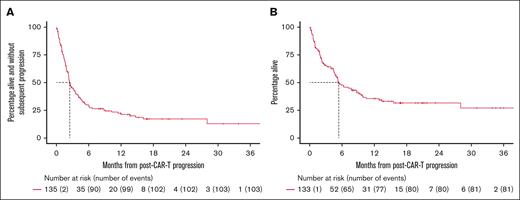
The median follow-up time from POD is 16 months among survivors (95% CI, 13-20). The median PFS and OS from POD were 2.5 (95% CI, 2.2-3.6) and 5.4 months (95% CI, 4.7-9.1), respectively, and the 1-year OS rate 36% (Figures 1 and 2).
Survival outcomes following post–CAR T-cell therapy POD. (A) PFS. (B) OS.
OS after POD according to time from CAR T-cell therapy infusion to POD.
Regarding predictors of OS after post–CAR T-cell therapy POD, age at diagnosis, deletion 17p, and age at CAR T-cell therapy infusion, time from CAR T-cell therapy infusion to POD, and lack of response to CAR T-cell therapy all conferred inferior OS in univariate analyses (Tables 4 and 5) after adjustments for multiple hypothesis testing.
Discussion
Our data, examining the largest reported cohort of patients with R/R MCL with POD post–CAR T-cell therapy, confirm the poor prognosis of this patient population, with a median OS of 5.4 months from POD. Adverse disease features were common pre–CAR T-cell therapy and at the time of POD. For patients receiving systemic therapy at the time of POD, BsAbs showed particular promise, with an ORR of 67%.
CD19-directed CAR T-cell therapy is a major advancement in treating R/R MCL; however, most patients’ disease ultimately progress following an initial response. For example, the median PFS in the registrational study of brexu-cel was 25.8 months,3 and liso-cel 15.3 months.2 In our cohort, most patients’ disease (83%) initially responded to CAR T-cell therapy, and POD post–CAR T-cell therapy occurred at a median of 6 months following infusion, with 27% experiencing POD at >12 months. In current practice, most patients with R/R MCL will likely be considered for CAR T-cell therapy at some point in their disease trajectory, and therefore understanding how patients are managed for POD post–CAR T-cell therapy represents a critical knowledge gap for the field. Our results roughly align with the only previous study in this area published by Jain and colleagues in 2021.5 In a small cohort of patients (N = 6), they reported a median OS from POD of 4.1 months, and a 12-month OS rate of 0%. Of note, this study occurred before more common use of BsAbs and the noncovalent BTKi pirtobrutinib. Other similar reports exist demonstrating poor outcomes achieved after POD for patients with diffuse large B-cell lymphoma,8-10 and emerging data show clear efficacy for BsAb in this clinical setting.11 Our data reinforce the exceptionally challenging outcomes observed in this population, and set a critical baseline for future investigations in this specific patient group.
Unsurprisingly, adverse disease features were common in our patient population both pre–CAR T-cell therapy and at the time of POD, including high rates of blastoid/pleomorphic morphology, somatic TP53 alterations, high Ki67, and refractoriness to prior therapy. This finding corroborates that many patients with R/R MCL receiving CAR T-cell therapy are heavily pretreated, and present with challenging disease biology. Although such patients’ disease can initially respond to CAR T-cell therapy, short subsequent survival from time of POD is still observed. Numerous clinical, disease, and treatment-related factors are associated with inferior OS after POD in univariate analyses, including advanced age (at diagnosis and at time of POD), deletion 17p identified pre–CAR T-cell therapy, lack of response to CAR T-cell therapy, and short time interval from CAR T-cell therapy infusion to POD. The presence of these characteristics should alert clinicians to patients at high risk for poor outcomes, and potentially inform treatment discussions.
A majority of patients, 78%, received systemic therapy at the time of POD, with the remaining patients undergoing local therapy (excision, radiation) or no further lymphoma-directed therapy. Although the number of patients in each systemic treatment category was small as practices were varied, it is encouraging that BsAb use was associated with an ORR of 67% and a median DOR of 10.5 months, albeit with limited follow-up. This class of agents is under active investigation as single agents, and in combination with other therapies active in treating R/R MCL. Phillips and colleagues reported results from a phase ½ study of the CD20-CD3 BsAb glofitamab in R/R MCL with an ORR of 85%; however, only 2 of 60 patients had received CAR T-cell therapy prior to enrollment. We await further results from BsAb-based studies12 to confirm activity in R/R MCL, and ultimately inform their use in practice.
Another noteworthy category was combinations of targeted therapies with 4 of 6 patients’ disease responding (ORR 67%); many such combinations included the B-cell lymphoma 2 inhibitor venetoclax. This substantiates published evidence supporting the use of venetoclax for R/R MCL, typically with an anti-CD20 monoclonal antibody or other targeted agents with the potential for synergistic activity.13,14 Our data suggest that combination approaches may be most appropriate in this setting for suitable patients to maximize the chances of response in the face of adverse disease features. Finally, our data concerning use of consolidative allo-HCT are noteworthy, as 6 of 8 such patients remain alive without disease, recognizing that such patients are likely younger and fitter than those not pursuing allo-HCT. For patients who achieve a remission with therapies post–CAR T-cell therapy and are suitable candidates for allo-HCT, this is a potentially curative option that should be considered in this high-risk context. Previous studies have demonstrated the efficacy of allo-HCT in R/R MCL even with high-risk features.15,16
There are limitations to our analysis that warrant mentioning. First, response criteria were not uniform across sites and, as with other data points, were missing in some instances at the different sites in our analysis. Second, as most patients received brexu-cel, we were unable to determine any effect of CAR T-cell therapy product on subsequent outcomes. Third, the response rates to some categories of treatments may reflect fitter patients eligible for clinical trials, and therefore response rates in a nontrial setting may be lower. Additionally, formal comparisons of efficacy across treatment categories are not possible due to limited numbers of patients in each group, and the retrospective nature of our analysis. Finally, not all patients underwent confirmatory biopsies at time of POD, thus somewhat limiting our understanding of the disease features at POD and how they might contribute to later outcomes after POD.
In conclusion, patients with R/R MCL experiencing POD following CAR T-cell therapy experience extremely poor outcomes in current practice. These patients should be strongly prioritized for new treatment approaches, potentially with BsAbs or other combination approaches in prospective, multicenter, collaborative clinical trials. Whether a subsequent CAR T-cell therapy infusion may be administered in those whose disease previously responded is an outstanding question. Patients with responding disease who are appropriate for allo-HCT should be engaged in such discussions early at the time of POD. Maintenance therapy administered post–CAR T-cell therapy may delay subsequent POD, especially in high-risk patients, and is also worthy of study. Additionally, whether earlier use of CAR T-cell therapy in patients’ disease courses may result in more durable responses, potentially due to improved T-cell fitness, is currently being investigated (NCT05495464, NCT06482684). Finally, the biologic underpinnings for CAR T-cell therapy failure in R/R MCL must be comprehensively defined to understand mechanisms of resistance, and to identify new therapeutic targets to ultimately improve outcomes for this urgent unmet need.
Acknowledgments
The authors acknowledge study staff at each site who contributed to data collection.
Z.D.E.-P. is supported by the Lymphoma Research Foundation, the Leukemia and Lymphoma Society and institutional P30 National Cancer Institute grant P30CA008748, the MSK Department of Medicine Internal grant, and Society for Memorial Sloan Kettering. A.K. is a Scholar in Clinical Research supported by Blood Cancer United.
Authorship
Contribution: Z.D.E.-P., P.J., and A.K. conceived of this study; Z.D.E.-P. wrote the manuscript; A.J. and E.D. performed statistical analyses; and all other authors contributed data, and reviewed and approved the manuscript.
Conflict-of-interest disclosure: Z.D.E.-P. participated in advisory board for Genmab; and performed educational programming for OncLive and Biopharm Communications. R.K. participated in advisory boards for AstraZeneca, Bristol Myers Squibb (BMS), Genentech/Roche, AbbVie, and Kite/Gilead; and in speakers bureau for BeiGene, AstraZeneca, and BMS. P.A.R. has served as a consultant and/or advisory board member for AbbVie, Novartis, BMS, ADC Therapeutics, Kite/Gilead, Pfizer, CVS Caremark, Genmab, BeiGene, Janssen, Pharmacyclics, and Genentech/Roche; received honoraria from Adaptive Biotechnologies; and received research support from BMS, Kite Pharma, Novartis, CRISPR Therapeutics, Calibr, Xencor, Fate Therapeutics, AstraZeneca, Genentech/Roche, Cellectis, Cargo Therapeutics, and Tessa Therapeutics. G.S. has received in the last 12 months financial compensations for participating in advisory boards for AbbVie, BeiGene, BMS, Genentech/Roche, Genmab, Janssen, Kite/Gilead, Merck, Novartis, Pfizer, Incyte, and Ipsen; and has received research support from AbbVie, Genentech, Genmab Janssen, Ipsen, and Nurix, which was managed by his institution. J.R. has provided consultancy services to AbbVie, AstraZeneca, ADC Therapeutics, BeiGene, BMS, Epizyme, Genentech, Genmab, Loxo Oncology, Janssen, Johnson and Johnson, MorphoSys, Pharmacyclics, and Pfizer; declares membership on a board or advisory committee for AbbVie, BeiGene, and Janssen; has received honoraria from Aptitude, Curio Science, MJH Life Sciences, and Ideology Health; and has received research funding from Acerta, AbbVie, BeiGene, Epizyme, Janssen, Loxo Oncology, Oncternal, Pharmacyclics, VelosBio, and Merck. M.J.F. consults for Kite Pharma–Gilead, Adaptative Biotechnologies, ADC Therapeutics, and Cargo Therapeutics; has received research support from Kite Pharma–Gilead, Allogene Therapeutics, Cargo Therapeutics, and Adaptative Biotechnologies; and is on the data safety monitoring board for Fate Therapeutics. K.J.M. has provided consulting services for AbbVie, ADC Therapeutics, AstraZeneca/Acerta, Autolus, BeiGene, BMS, Caribou, Genentech, Genmab, Gilead/Kite, Incyte, Janssen, Lilly, MorphoSys, and Pharmacyclics. A.I. has provided consulting services for and received honoraria from Genmab, Seagen, Gilead/Kite, and AstraZeneca. Y.W. has received research funding (to institution) from Incyte, InnoCare, Loxo Oncology, Eli Lilly, MorphoSys, Novartis, Genentech, Genmab, AbbVie, BeiGene, and Merck; has participated in advisory boards (compensation to institution) for Eli Lilly, Loxo Oncology, TG Therapeutics, Incyte, InnoCare, Kite, Jansen, BeiGene, AstraZeneca, Genmab, and AbbVie; has provided consultancy services (compensation to institution) for InnoCare and AbbVie; and has received honorarium (to institution) from Kite. M.K.K. has received research support/funding from Novartis; has provided consultancy services to AbbVie, AstraZeneca, Celgene/BMS, BeiGene, and Genentech; and has served on data monitoring committee for Celgene and Genentech. P.J. declares receiving lymphoma research foundation CDA award grant; participating on advisory boards for Eli Lilly, Kite, AstraZeneca, Loxo Oncology, Incyte, Janssen-PCYC, and Kite; receiving honorarium from Aptitude Health, Pharmacy Times, Dava Oncology, Adaptive Biotech, Eli Lilly, BeiGene, and Genentech; and receiving research funding from AstraZeneca, Kite, BeiGene, and Genentech. C.E.R. has received consulting fees from AstraZeneca and Genentech; and has received institutional research funding from BeiGene, Genentech, and Lilly. The remaining authors declare no competing financial interests.
Correspondence: Zachary Epstein-Peterson, Memorial Sloan Kettering Cancer Center, 530 E 74th St, New York, NY 10021; email: epsteinz@mskcc.org.
References
Author notes
P.J. and A.K. contributed equally to this study.
Presented in oral abstract form at the 66th annual meeting of the American Society of Hematology, San Diego, CA, 7 December 2024.
Data are available on request from the corresponding author, Zachary Epstein-Peterson (epsteinz@mskcc.org).
The full-text version of this article contains a data supplement.