In this issue of Blood Advances, de Boer et al1 validate the predictive capacity of the CAR-HEMATOTOX (HT) as a risk model for cytopenias following chimeric antigen receptor T-cell therapy (CAR-T), now termed immune effector cell–associated hematotoxicity (ICAHT),2 as well as for infections and survival. To this end, the authors performed a population-based retrospective study spanning 7 adult CAR-T centers in the Netherlands, comprising a cohort of 245 patients with relapsed/refractory large B-cell lymphoma treated with standard-of-care axicabtagene ciloleucel. As the primary end point, the authors explored associations between continuous or binary HT scores and clinically significant neutropenia, defined as severe neutropenia for at least 14 days postinfusion. Subsequently, the performance of the HT score as a predictor of grade 3 or higher early (day 0-30) and late (day >30) ICAHT and severe infection events, was assessed. Finally, de Boer et al compared progression-free survival (PFS) and overall survival (OS) by HT risk groups. The authors are to be lauded for the compilation and analysis of this granular data set, which can be regarded as a relevant contribution to the field of CAR-T toxicity research and risk prediction and positions the HT score closer to routine clinical application.
As previously described,3 the authors determined the HT score using the absolute neutrophil count, hemoglobin level, platelet count, and C-reactive protein (CRP) and ferritin levels before lymphodepletion. For further analysis, patients were allocated to either the HTlow (score 0-1; 30%) or HThigh (score ≥2; 70%) risk groups. The proportion of high-risk patients was thus slightly higher than that in the original publication (pooled analysis, 49%), reflecting a heavily pretreated real-world cohort.3 Notably, a complete laboratory profile for the exact determination of the HT score was available for 142 patients. To address the challenge of missing score parameters (a frequent issue in clinical routine especially for serum inflammatory markers such as CRP and ferritin), the authors developed a novel R code based on multiple imputation by chained equations, thereby providing a valuable resource to the community for further scientific research and application of the risk model in larger patient cohorts (full R script found in the supplemental Methods).
Clinically significant neutropenia affected 55 patients (22%), translating to a rate of 20% grade ≥3 early ICAHT according to the European Hematology Association (EHA) / European Society for Blood and Marrow Transplantation (EBMT) grading2 and highlighting the continued relevance of this side effect. Importantly, the authors reported a significant association between severe ICAHT and both the binary (odds ratio [OR], 2.94; P = .01) and continuous (OR, 1.78; P < .001) HT score, with an area under the curve of 0.73. The associations were also noted when testing the individual continuous laboratory parameters in univariate models. Therefore, we share the authors’ opinion that the HT score was confirmed to be a valuable resource for the identification of patients at risk for protracted neutropenia.
ICAHT represents the most common adverse effect of CAR-T and can predispose patients to infectious complications, which represent the principal driver of nonrelapse mortality in CAR-T recipients.4,5 Therefore, the authors tested associations between the HT score and severe infections. Statistical significance was reached for the continuous (OR, 1.26; P = .027) but not for the binary HT score using a cutoff of 2 (OR, 2.02; P = .085), although a numerically higher rate of severe infection events was noted for patients at this threshold (24% vs 12%). As discussed by the authors, the observed results have to be interpreted under careful consideration of improved toxicity management strategies over time, including increased rates of granulocyte colony-stimulating factor application in the HThigh group and the multifactorial etiology of infections.6
Interestingly, the authors observed inferior PFS (hazard ratio [HR], 1.84; P = .01) and OS (HR, 2.83; P<.001) among HThigh patients, as well as a trend toward an increased incidence of nonrelapse mortality (P = .073). These results emphasize the prognostic significance of systemic inflammation and bone marrow reserve before CAR-T initiation. The host inflammatory state plays a key role in shaping subsequent CAR T-cell expansion in patients with lymphoma7 and often reflects other poor risk features such as adverse tumor genomics and an immunohostile microenvironment.8 Furthermore, baseline cytopenias may indicate the extent of bone marrow disease burden (in patients with underlying marrow involvement) or hint at impaired underlying T-cell fitness.9 Further studies are ultimately needed to address the question whether the combination with other established clinical risk factors can further refine prognostic assessments, with the overall goal not only to identify critical patients but also to provide them with concrete treatment recommendations (eg, combinatorial strategies or clinical trials of novel agents).
The limitations of this study arise from the moderate patient numbers, restriction to CAR-T centers in the Netherlands, and absence of data with other CAR-T constructs, particularly the frequently applied lisocabtagene maraleucel. Another limitation relates to the fact that more detailed analyses of supportive measures, such as transfusions or IgG replacement, and of the longitudinal recovery of platelet count and hemoglobin values were not part of the study. In addition, multivariable models incorporating other relevant patient- and disease-related characteristics, such as serum lactate dehydrogenase or metabolic tumor volume, were not performed.
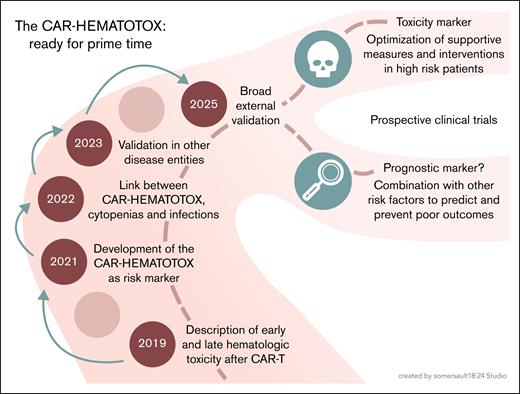
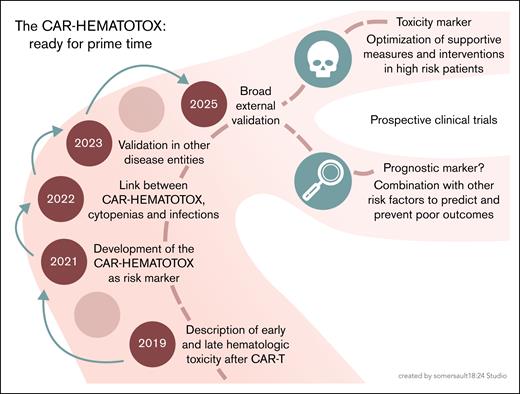
Despite the mentioned limitations, the study conducted by de Boer et al supports the use of the HT score as a simple and reliable tool to identify vulnerable patients at an increased risk for severe cytopenias, associated complications including life-threatening infections, and inferior treatment outcomes. Accordingly, this robust external validation in a large population–based cohort, together with other recent studies,10 firmly establishes the HT score in clinical routine. However, the journey and open questions do not end here (see figure). The approval criteria for CAR-T products continue to evolve, with clinical trials providing increasing evidence for the use of CAR-T at earlier disease stages across hematological malignancies. Another trend is the use of CAR T cells in an increasingly broad spectrum of solid tumor entities and autoimmune diseases. In these novel settings, it will be necessary to reexamine the relevance of established risk markers and adapt them, if necessary.
From development to clinical application of the CAR-HEMATOTOX score: an overview of its evolution, validation, and future directions. Professional illustration by Somersault18:24.
From development to clinical application of the CAR-HEMATOTOX score: an overview of its evolution, validation, and future directions. Professional illustration by Somersault18:24.
The ultimate clinical utility of risk scores such as the HT will be derived from their ability to sufficiently guide supportive strategies that make CAR-T more effective and safer for high-risk patients. Measures to prevent and treat cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome have long been the focus of clinical studies.11 However, mitigation strategies for ICAHT and infections remain understudied, despite being the most common adverse events and major drivers of morbidity and mortality.12 The optimization of tumor control before starting lymphodepletion, closer follow-up intervals, intensified infection prophylaxis, immunoglobulin replacement, the early use of growth factors, and stem cell boosts are only a few examples for possible starting points to counteract and attenuate this toxicity. Prospective clinical trials will help identify the most effective approaches and patients who benefit most, and future studies should evaluate the HT score as a clinical guide for the allocation of targeted measures. The end points of studies on CAR-T–related toxicities should include not only current grading systems for ICAHT2,13 but also the resulting clinical consequences, such as transfusion dependency, severe infections, and inferior quality of life.
In summary, investigation of the HT and other scores across entities, CAR-T constructs, time points, and potentially other types of immunotherapies will help to identify the optimal context and tools for risk stratification, with the overall goal to improve patient safety. Future studies should identify optimal prevention and management strategies for post-CAR-T cytopenias and infections, and prospectively apply the HT score as a guide for clinical decision-making.
Conflict-of-interest disclosure: J.H.F. has an advisory role with Pfizer; reports honoraria from Bristol Myers Squibb and Stemline; and travel and congress participation grants from Janssen-Cilag. K.R. reports research funding, consultancy fees, honoraria, and travel support from Kite/Gilead; honoraria from Novartis; consultancy fees and honoraria from Bristol Myers Squibb/Celgene; travel support from Pierre Fabre; and consultancy fees from CSL Behring.